Regulation of BRCA1 stability through the tandem UBX domains of isoleucyl-tRNA synthetase 1.
Chung, S., Kang, M.S., Alimbetov, D.S., Mun, G.I., Yunn, N.O., Kim, Y., Kim, B.G., Wie, M., Lee, E.A., Ra, J.S., Oh, J.M., Lee, D., Lee, K., Kim, J., Han, S.H., Kim, K.T., Chung, W.K., Nam, K.H., Park, J., Lee, B., Kim, S., Zhao, W., Ryu, S.H., Lee, Y.S., Myung, K., Cho, Y.(2022) Nat Commun 13: 6732-6732
- PubMed: 36347866
- DOI: https://doi.org/10.1038/s41467-022-34612-y
- Primary Citation of Related Structures:
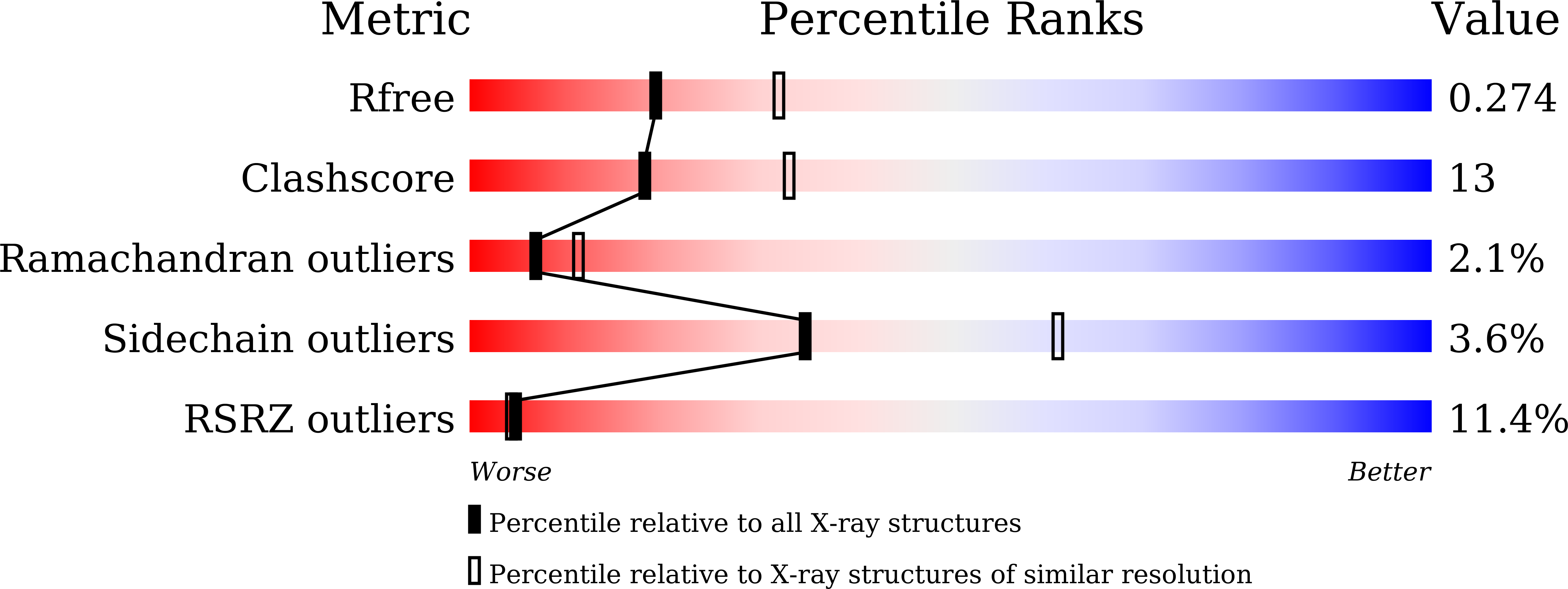
7WRS, 7WRU - PubMed Abstract:
Aminoacyl-tRNA synthetases (ARSs) have evolved to acquire various additional domains. These domains allow ARSs to communicate with other cellular proteins in order to promote non-translational functions. Vertebrate cytoplasmic isoleucyl-tRNA synthetases (IARS1s) have an uncharacterized unique domain, UNE-I. Here, we present the crystal structure of the chicken IARS1 UNE-I complexed with glutamyl-tRNA synthetase 1 (EARS1). UNE-I consists of tandem ubiquitin regulatory X (UBX) domains that interact with a distinct hairpin loop on EARS1 and protect its neighboring proteins in the multi-synthetase complex from degradation. Phosphomimetic mutation of the two serine residues in the hairpin loop releases IARS1 from the complex. IARS1 interacts with BRCA1 in the nucleus, regulates its stability by inhibiting ubiquitylation via the UBX domains, and controls DNA repair function.
Organizational Affiliation:
Department of Life Sciences, Pohang University of Science and Technology, Pohang, Republic of Korea.