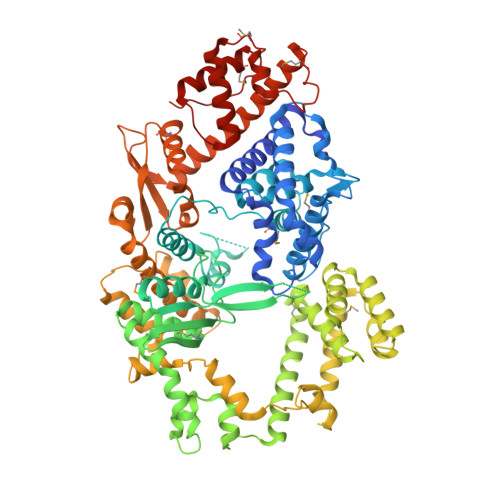
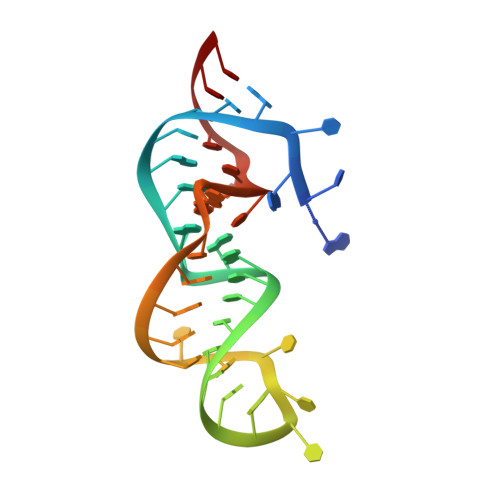
Structure and engineering of the minimal type VI CRISPR-Cas13bt3.
Nakagawa, R., Kannan, S., Altae-Tran, H., Takeda, S.N., Tomita, A., Hirano, H., Kusakizako, T., Nishizawa, T., Yamashita, K., Zhang, F., Nishimasu, H., Nureki, O.(2022) Mol Cell 82: 3178-3192.e5
- PubMed: 36027912
- DOI: https://doi.org/10.1016/j.molcel.2022.08.001
- Primary Citation of Related Structures:
7VTI, 7VTN - PubMed Abstract:
Type VI CRISPR-Cas13 effector enzymes catalyze RNA-guided RNA cleavage and have been harnessed for various technologies, such as RNA detection, targeting, and editing. Recent studies identified Cas13bt3 (also known as Cas13X.1) as a miniature Cas13 enzyme, which can be used for knockdown and editing of target transcripts in mammalian cells. However, the action mechanism of the compact Cas13bt3 remains unknown. Here, we report the structures of the Cas13bt3-guide RNA complex and the Cas13bt3-guide RNA-target RNA complex. The structures revealed how Cas13bt3 recognizes the guide RNA and its target RNA and provided insights into the activation mechanism of Cas13bt3, which is distinct from those of the other Cas13a/d enzymes. Furthermore, we rationally engineered enhanced Cas13bt3 variants and ultracompact RNA base editors. Overall, this study improves our mechanistic understanding of the CRISPR-Cas13 enzymes and paves the way for the development of efficient Cas13-mediated transcriptome modulation technologies.
- Department of Biological Sciences, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
Organizational Affiliation: