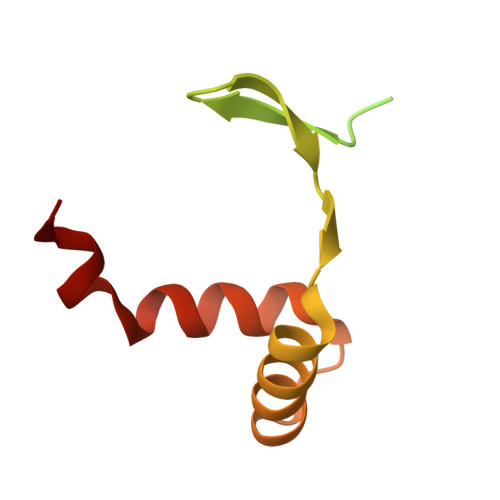
DNA-TCP complex structures reveal a unique recognition mechanism for TCP transcription factor families.
Zhang, Y., Xu, Y.P., Nie, J.K., Chen, H., Qin, G., Wang, B., Su, X.D.(2023) Nucleic Acids Res 51: 434-448
- PubMed: 36546761
- DOI: https://doi.org/10.1093/nar/gkac1171
- Primary Citation of Related Structures:
7VP1, 7VP2, 7VP3, 7VP4, 7VP5, 7VP6, 7VP7 - PubMed Abstract:
Plant-specific TCP transcription factors are key regulators of diverse plant functions. TCP transcription factors have long been annotated as basic helix-loop-helix (bHLH) transcription factors according to remote sequence homology without experimental validation, and their consensus DNA-binding sequences and protein-DNA recognition mechanisms have remained elusive. Here, we report the crystal structures of the class I TCP domain from AtTCP15 and the class II TCP domain from AtTCP10 in complex with different double-stranded DNA (dsDNA). The complex structures reveal that the TCP domain is a distinct DNA-binding motif and the homodimeric TCP domains adopt a unique three-site recognition mode, binding to dsDNA mainly through a central pair of β-strands formed by the dimer interface and two basic flexible loops from each monomer. The consensus DNA-binding sequence for class I TCPs is a perfectly palindromic 11 bp (GTGGGNCCCAC), whereas that for class II TCPs is a near-palindromic 11 bp (GTGGTCCCCAC). The unique DNA binding mode allows the TCP domains to display broad specificity for a range of DNA sequences even shorter than 11 bp, adding further complexity to the regulatory network of plant TCP transcription factors.
- State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences, and Biomedical Pioneering Innovation Center (BIOPIC), Peking University, Beijing, 100871, China.
Organizational Affiliation: