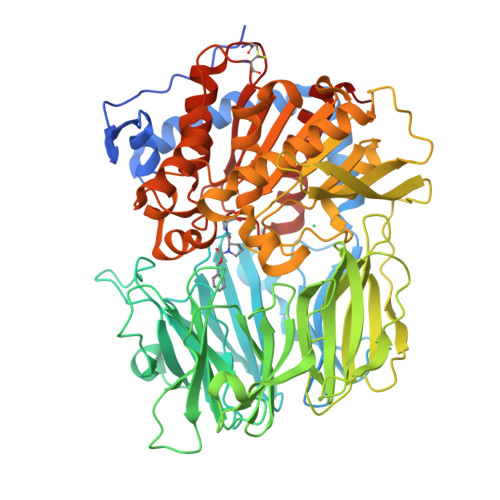
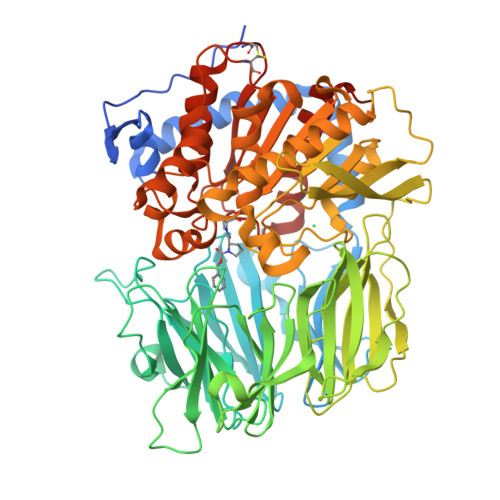
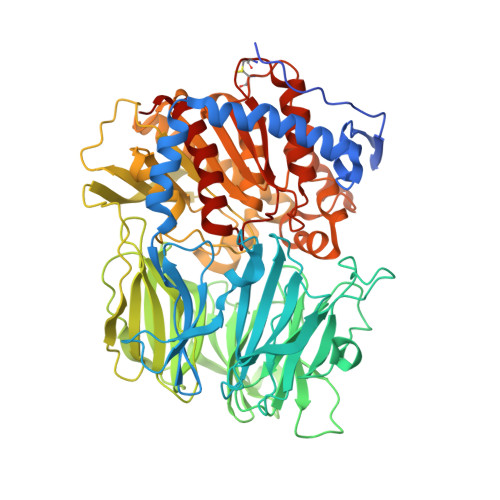
The structure and molecular dynamics of prolyl oligopeptidase from Microbulbifer arenaceous provide insights into catalytic and regulatory mechanisms.
Huang, P., Lv, A., Yan, Q., Jiang, Z., Yang, S.(2022) Acta Crystallogr D Struct Biol 78: 735-751
- PubMed: 35647921
- DOI: https://doi.org/10.1107/S2059798322004247
- Primary Citation of Related Structures:
7VGB, 7VGC - PubMed Abstract:
Prolyl oligopeptidases (POPs) are atypical serine proteases that are unique in their involvement in the maturation and degradation of prolyl-containing peptide hormones and neuropeptides. They are potential pharmaceutical targets for the treatment of several neurodegenerative disorders, such as Alzheimer's disease. In this study, the catalytic and substrate-regulatory mechanisms of a novel bacterial POP from Microbulbifer arenaceous (MaPOP) were investigated. The crystal structure revealed that the catalytic triad of MaPOP was covered by the central tunnel of an unusual β-propeller domain. The tunnel not only provided the sole access to the active site for oligopeptides, but also protected large structured peptides or proteins from accidental proteolysis. The enzyme was able to cleave angiotensin I specifically at the carboxyl side of the internal proline residue, but could not hydrolyze long-chain bovine insulin B in vitro. Like the ligand-free structure, MaPOP bound to the transition-state analog inhibitor ZPR was also in a closed state, which was not modulated by the common `latching loop' found in other POPs. The substrate-assisted catalytic mechanism of MaPOP reported here may represent a common mechanism for all POPs. These results may facilitate a better understanding of the catalytic behavior of POPs under physiological conditions.
Organizational Affiliation:
Beijing Advanced Innovation Center for Food Nutrition and Human Health and College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, People's Republic of China.