Preclinical development of ZED8, an 89 Zr immuno-PET reagent for monitoring tumor CD8 status in patients undergoing cancer immunotherapy.
Ogasawara, A., Kiefer, J.R., Gill, H., Chiang, E., Sriraman, S., Ferl, G.Z., Ziai, J., Bohorquez, S.S., Guelman, S., Wang, X., Yang, J., Phan, M.M., Nguyen, V., Chung, S., Yu, C., Tinianow, J., Waaijer, S.J.H., De Crespigny, A., Marik, J., Boswell, C.A., Zabka, T., Staflin, K., Williams, S.P.(2023) Eur J Nucl Med Mol Imaging 50: 287-301
- PubMed: 36271158
- DOI: https://doi.org/10.1007/s00259-022-05968-6
- Primary Citation of Related Structures:
7UVF - PubMed Abstract:
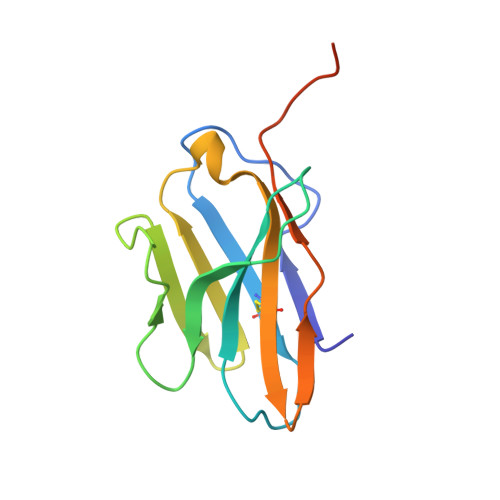
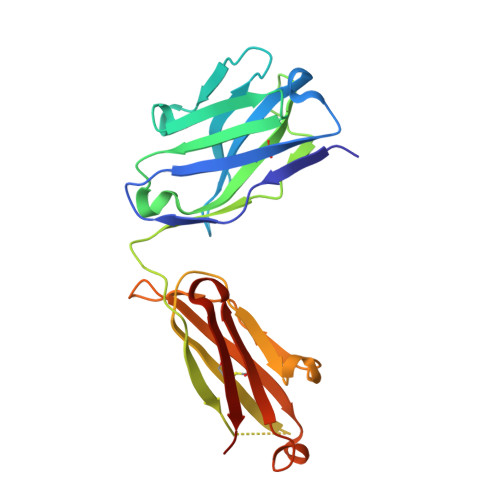
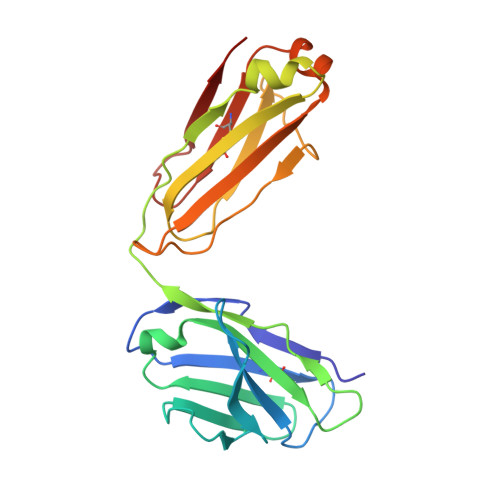
ZED8 is a novel monovalent antibody labeled with zirconium-89 for the molecular imaging of CD8. This work describes nonclinical studies performed in part to provide rationale for and to inform expectations in the early clinical development of ZED8, such as in the studies outlined in clinical trial registry NCT04029181 [1]. Surface plasmon resonance, X-ray crystallography, and flow cytometry were used to characterize the ZED8-CD8 binding interaction, its specificity, and its impact on T cell function. Immuno-PET with ZED8 was assessed in huCD8 + tumor-bearing mice and in non-human primates. Plasma antibody levels were measured by ELISA to determine pharmacokinetic parameters, and OLINDA 1.0 was used to estimate radiation dosimetry from image-derived biodistribution data. ZED8 selectively binds to human CD8α at a binding site approximately 9 Å from that of MHCI making mutual interference unlikely. The equilibrium dissociation constant (K D ) is 5 nM. ZED8 binds to cynomolgus CD8 with reduced affinity (66 nM) but it has no measurable affinity for rat or mouse CD8. In a series of lymphoma xenografts, ZED8 imaging was able to identify different CD8 levels concordant with flow cytometry. In cynomolgus monkeys with tool compound 89 Zr-aCD8v17, lymph nodes were conspicuous by imaging 24 h post-injection, and the pharmacokinetics suggested a flat-fixed first-in-human dose of 4 mg per subject. The whole-body effective dose for an adult human was estimated to be 0.48 mSv/MBq, comparable to existing 89 Zr immuno-PET reagents. 89 Zr immuno-PET with ZED8 appears to be a promising biomarker of tissue CD8 levels suitable for clinical evaluation in cancer patients eligible for immunotherapy.
- Department of Biomedical Imaging, Genentech, Inc, 1 DNA Way, South San Francisco, CA, 94080, USA.
Organizational Affiliation: