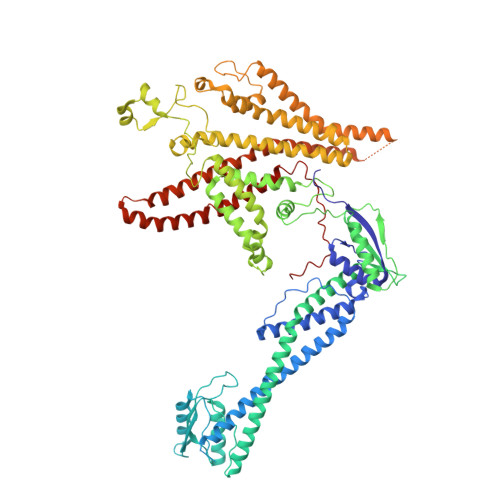
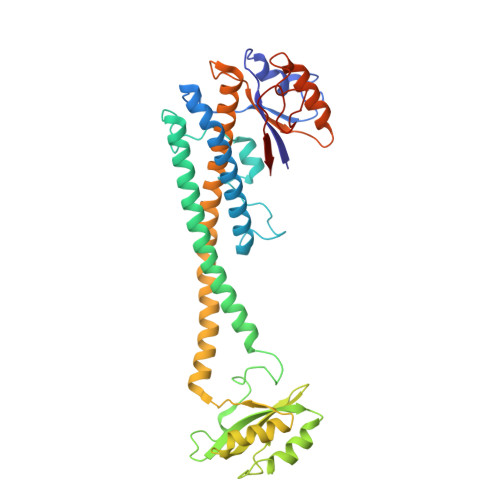
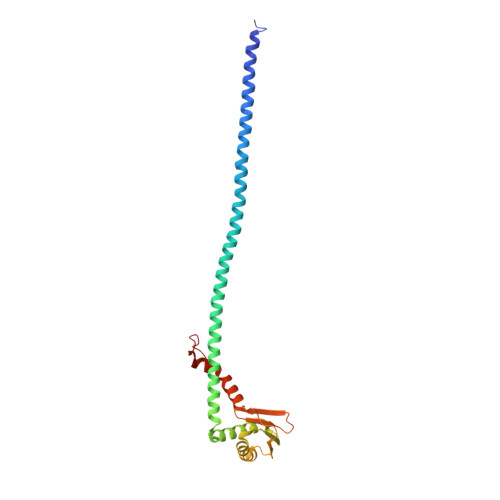
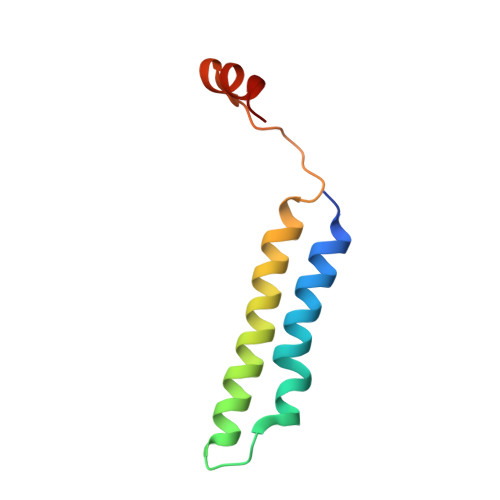
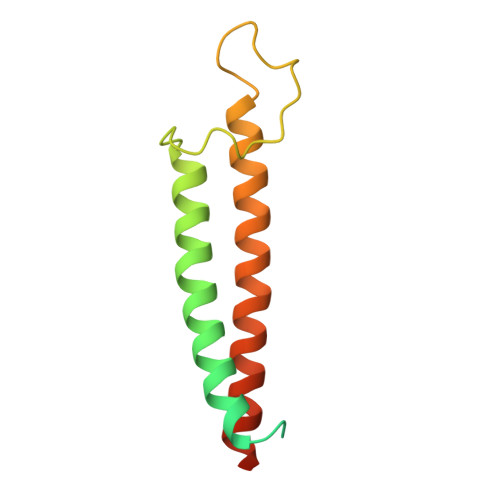
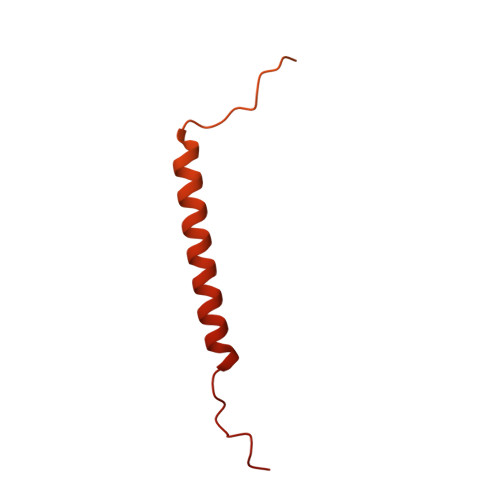
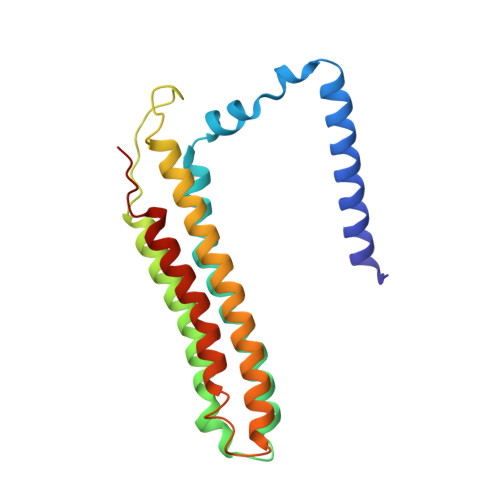
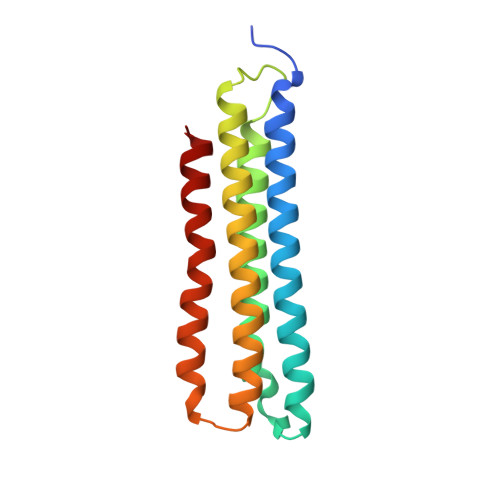
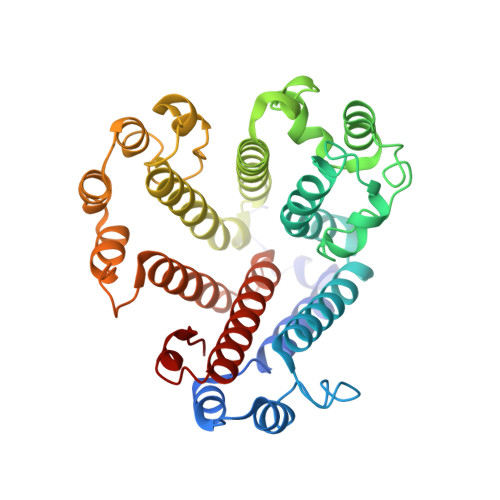
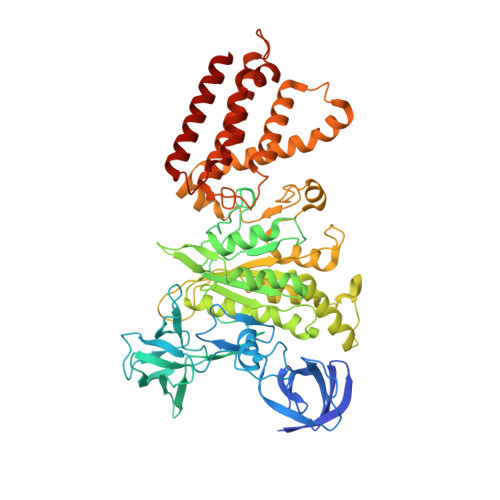
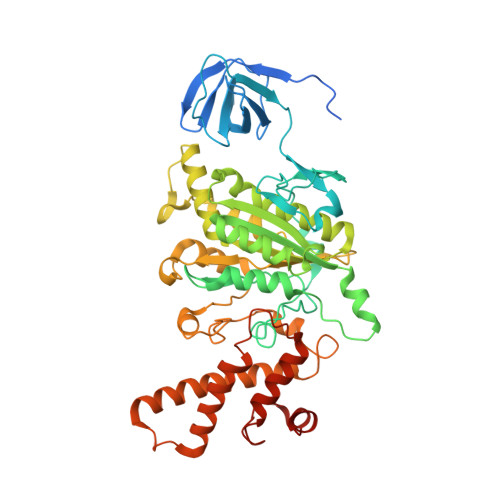
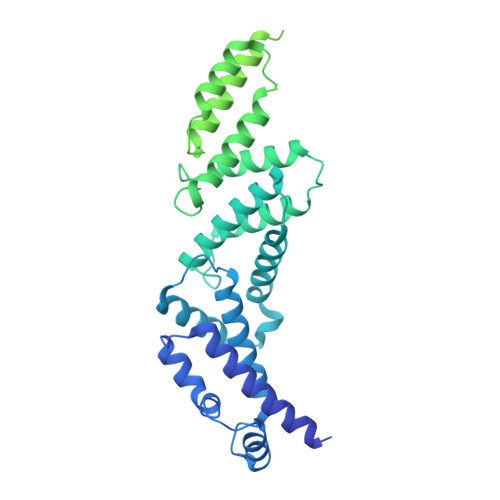
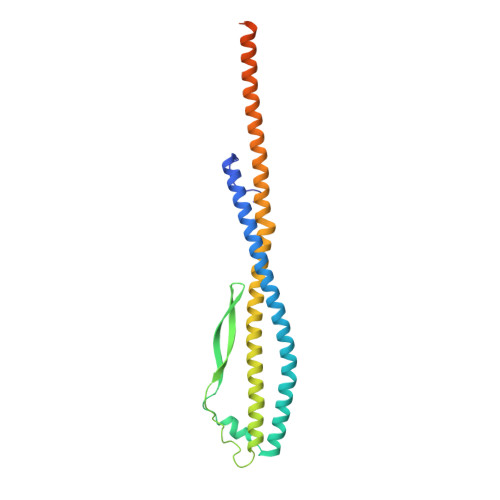
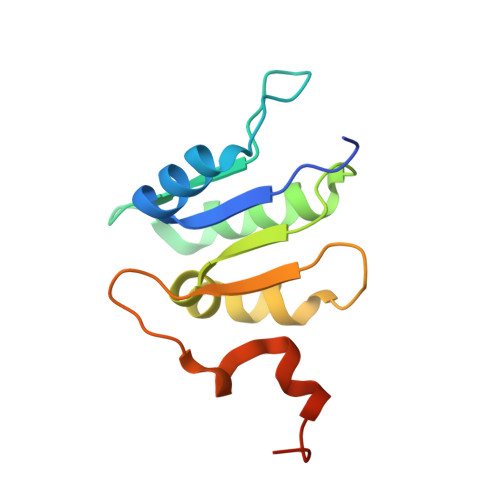
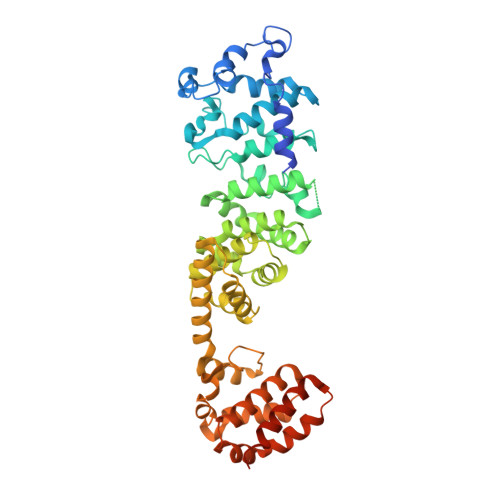
Identification of mEAK-7 as a human V-ATPase regulator via cryo-EM data mining.
Wang, L., Wu, D., Robinson, C.V., Fu, T.M.(2022) Proc Natl Acad Sci U S A 119: e2203742119-e2203742119
- PubMed: 35994636
- DOI: https://doi.org/10.1073/pnas.2203742119
- Primary Citation of Related Structures:
7U4T - PubMed Abstract:
Vacuolar-type adenosine triphosphatases (V-ATPases) not only function as rotary proton pumps in cellular organelles but also serve as signaling hubs. To identify the endogenous binding partners of V-ATPase, we collected a large dataset of human V-ATPases and did extensive classification and focused refinement of human V-ATPases. Unexpectedly, about 17% of particles in state 2 of human V-ATPases display additional density with an overall resolution of 3.3 Å. Structural analysis combined with artificial intelligence modeling enables us to identify this additional density as mEAK-7, a protein involved in mechanistic target of rapamycin (mTOR) signaling in mammals. Our structure shows that mEAK-7 interacts with subunits A, B, D, and E of V-ATPases in state 2. Thus, we propose that mEAK-7 may regulate V-ATPase function through binding to V-ATPases in state 2 as well as mediate mTOR signaling.
- Department of Cardiology, Zhongnan Hospital of Wuhan University, School of Pharmaceutical Sciences, Wuhan University, Wuhan 430071, China.
Organizational Affiliation: