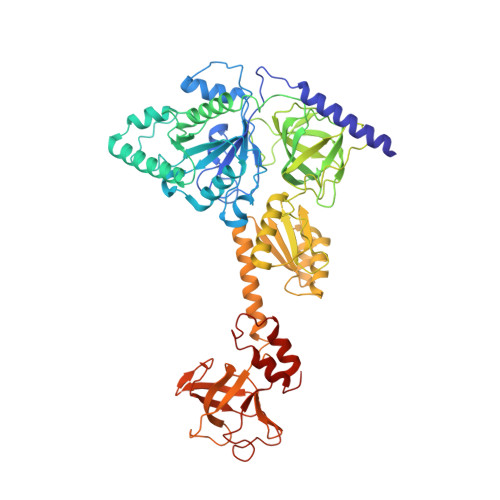
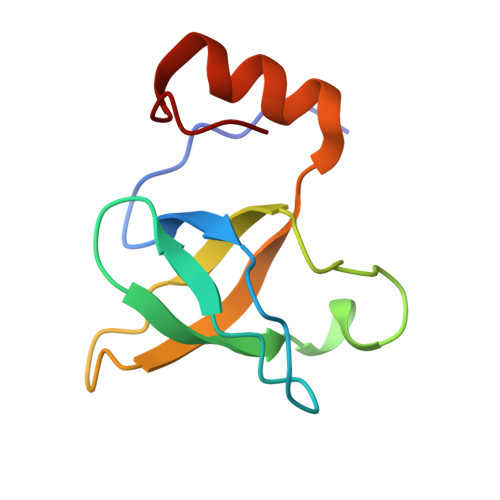
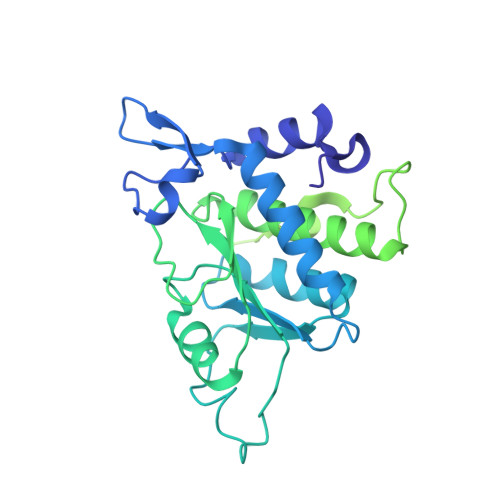
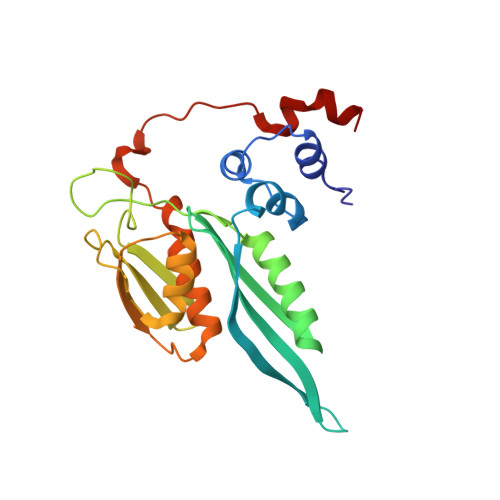
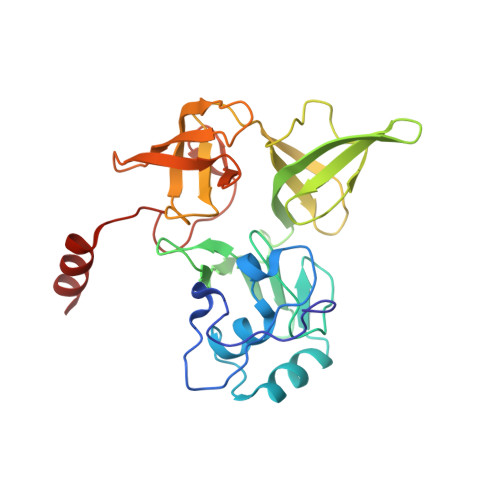
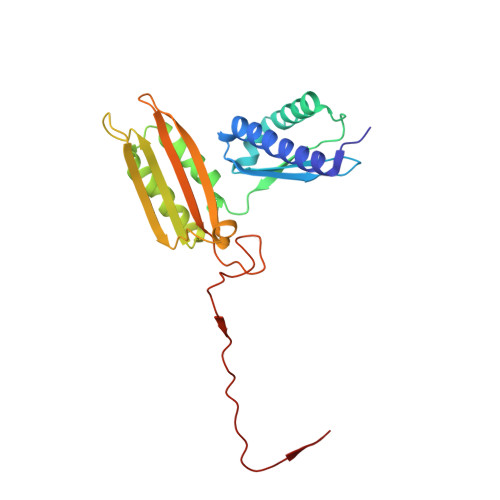
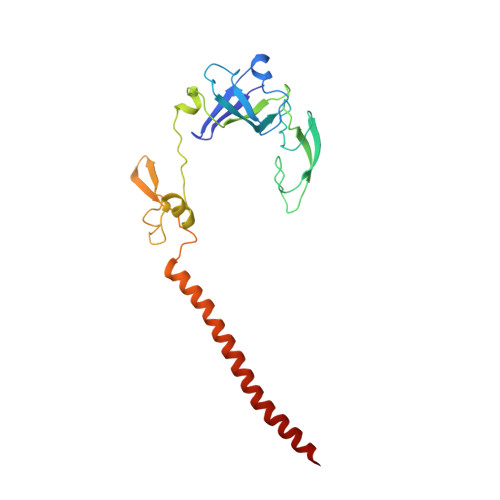
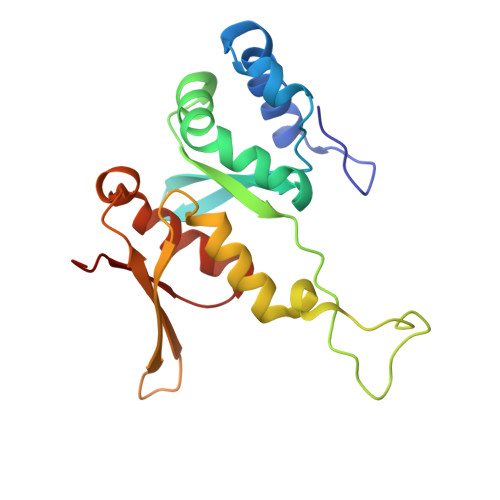
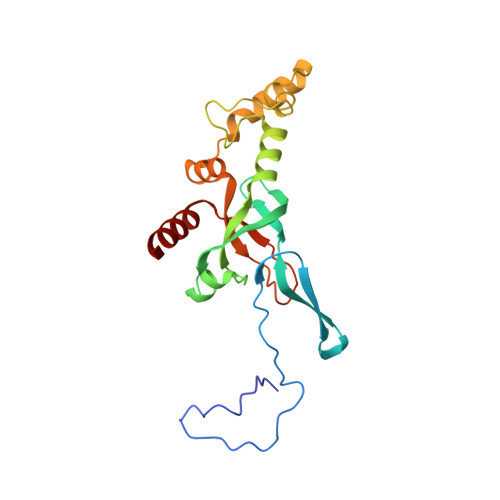
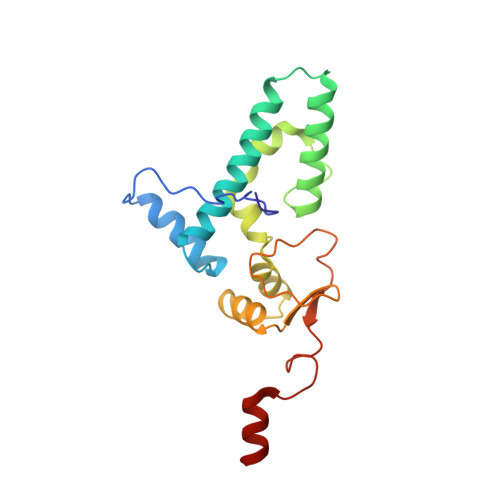
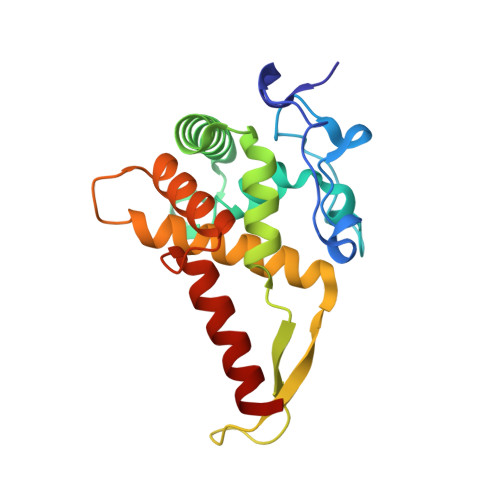
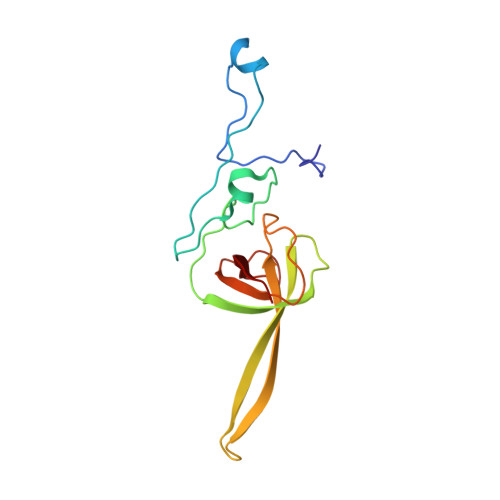
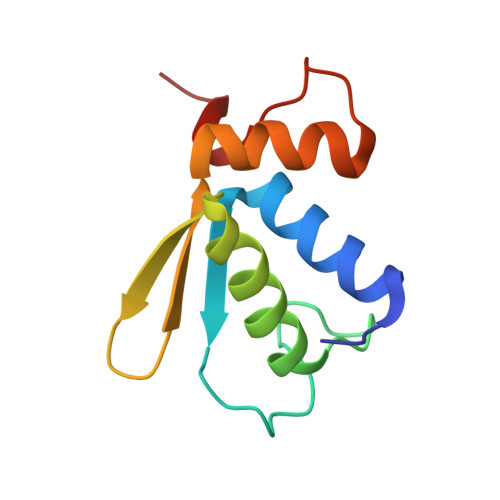
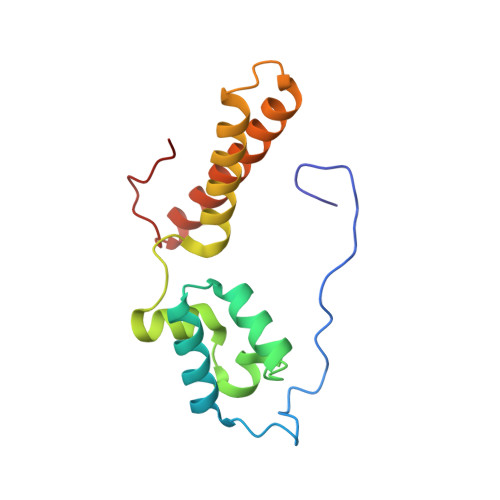
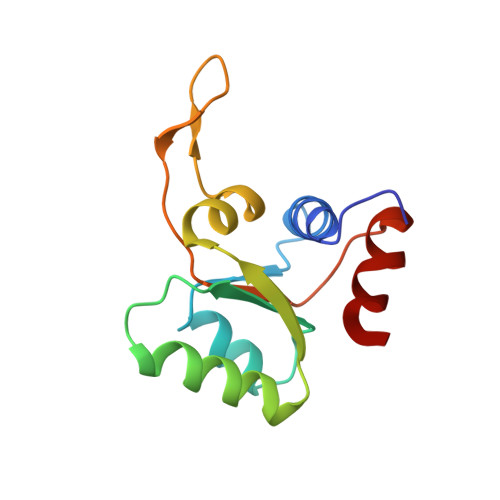
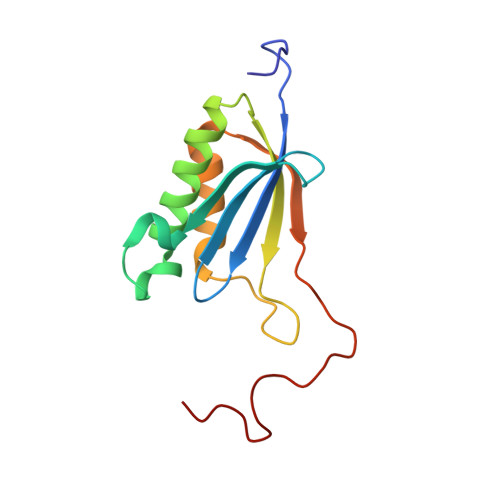
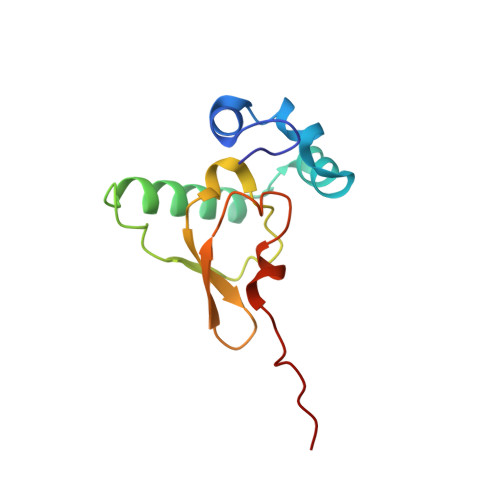
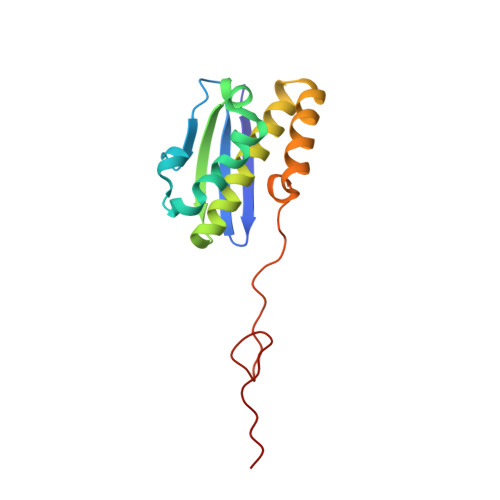
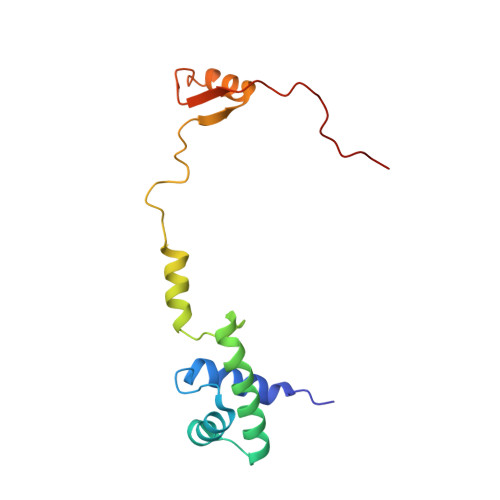
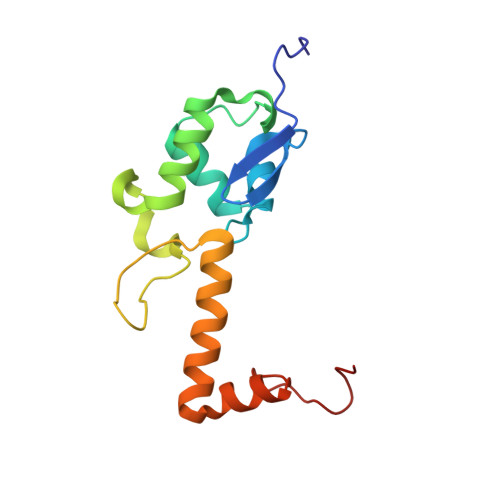
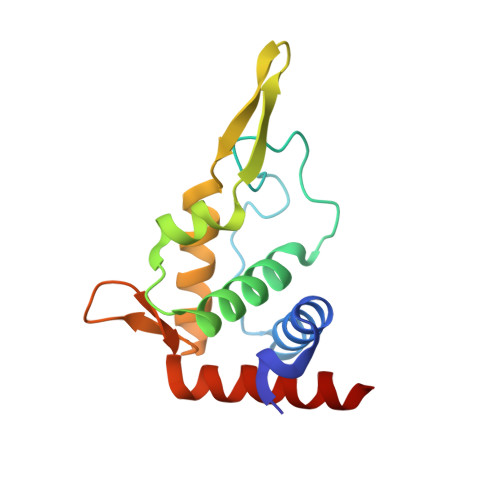
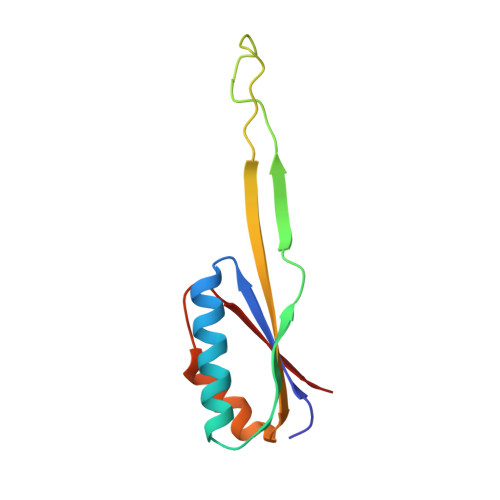
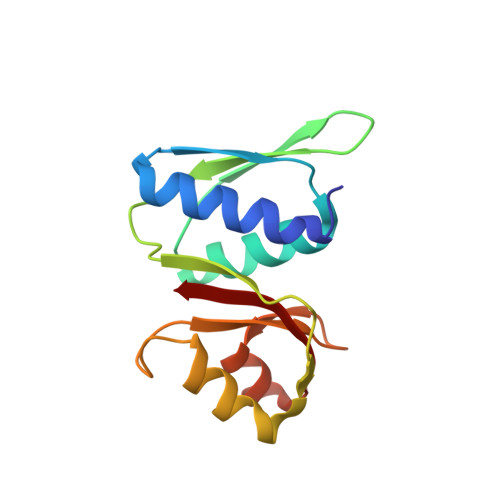
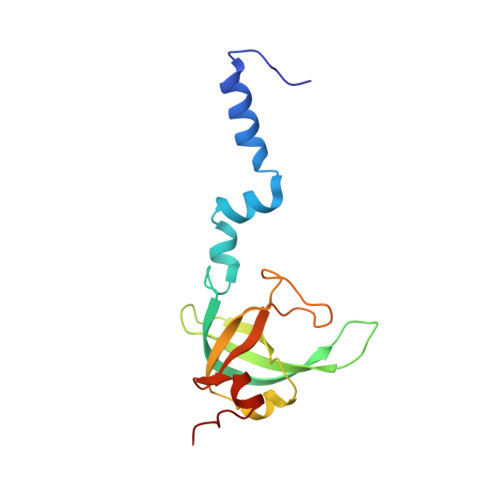
eIF5B and eIF1A reorient initiator tRNA to allow ribosomal subunit joining.
Lapointe, C.P., Grosely, R., Sokabe, M., Alvarado, C., Wang, J., Montabana, E., Villa, N., Shin, B.S., Dever, T.E., Fraser, C.S., Fernandez, I.S., Puglisi, J.D.(2022) Nature 607: 185-190
- PubMed: 35732735
- DOI: https://doi.org/10.1038/s41586-022-04858-z
- Primary Citation of Related Structures:
7TQL - PubMed Abstract:
Translation initiation defines the identity and quantity of a synthesized protein. The process is dysregulated in many human diseases 1,2 . A key commitment step is when the ribosomal subunits join at a translation start site on a messenger RNA to form a functional ribosome. Here, we combined single-molecule spectroscopy and structural methods using an in vitro reconstituted system to examine how the human ribosomal subunits join. Single-molecule fluorescence revealed when the universally conserved eukaryotic initiation factors eIF1A and eIF5B associate with and depart from initiation complexes. Guided by single-molecule dynamics, we visualized initiation complexes that contained both eIF1A and eIF5B using single-particle cryo-electron microscopy. The resulting structure revealed how eukaryote-specific contacts between the two proteins remodel the initiation complex to orient the initiator aminoacyl-tRNA in a conformation compatible with ribosomal subunit joining. Collectively, our findings provide a quantitative and architectural framework for the molecular choreography orchestrated by eIF1A and eIF5B during translation initiation in humans.
- Department of Structural Biology, Stanford University School of Medicine, Stanford, CA, USA.
Organizational Affiliation: