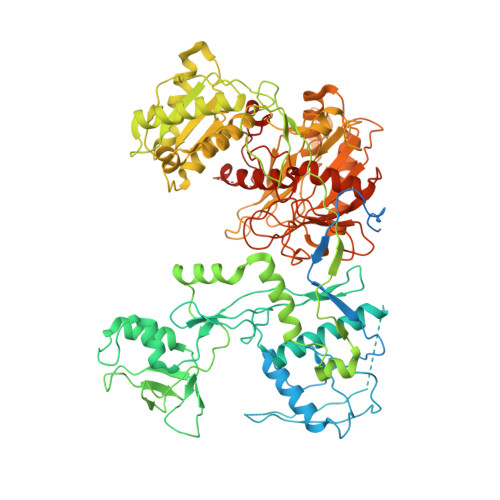
The molecular mechanism of microRNA duplex selectivity of Arabidopsis ARGONAUTE10.
Xiao, Y., MacRae, I.J.(2022) Nucleic Acids Res 50: 10041-10052
- PubMed: 35801914
- DOI: https://doi.org/10.1093/nar/gkac571
- Primary Citation of Related Structures:
7SWF - PubMed Abstract:
Small RNAs (sRNAs), including microRNAs (miRNAs) and small interfering RNAs (siRNAs), are essential gene regulators for plant and animal development. The loading of sRNA duplexes into the proper ARGONAUTE (AGO) protein is a key step to forming a functional silencing complex. In Arabidopsis thaliana, the specific loading of miR166/165 into AGO10 (AtAGO10) is critical for the maintenance of the shoot apical meristem, the source of all shoot organs, but the mechanism by which AtAGO10 distinguishes miR166/165 from other cellular miRNAs is not known. Here, we show purified AtAGO10 alone lacks loading selectivity towards miR166/165 duplexes. However, phosphate and HSP chaperone systems reshape the selectivity of AtAGO10 to its physiological substrates. A loop in the AtAGO10 central cleft is essential for recognizing specific mismatches opposite the guide strand 3' region in miR166/165 duplexes. Replacing this loop with the equivalent loop from Homo sapiens AGO2 (HsAGO2) changes AtAGO10 miRNA loading behavior such that 3' region mismatches are ignored and mismatches opposite the guide 5' end instead drive loading, as in HsAGO2. Thus, this study uncovers the molecular mechanism underlying the miR166/165 selectivity of AtAGO10, essential for plant development, and provides new insights into how miRNA duplex structures are recognized for sRNA sorting.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.
Organizational Affiliation: