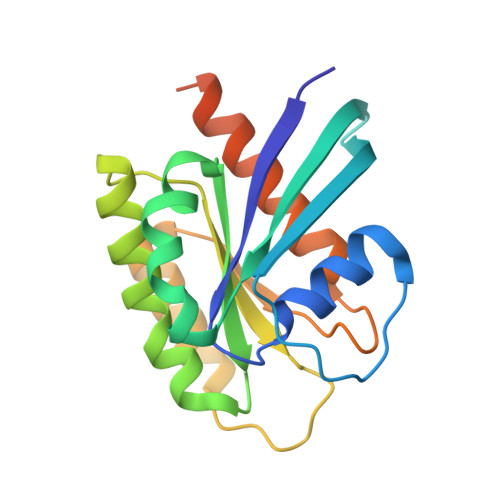
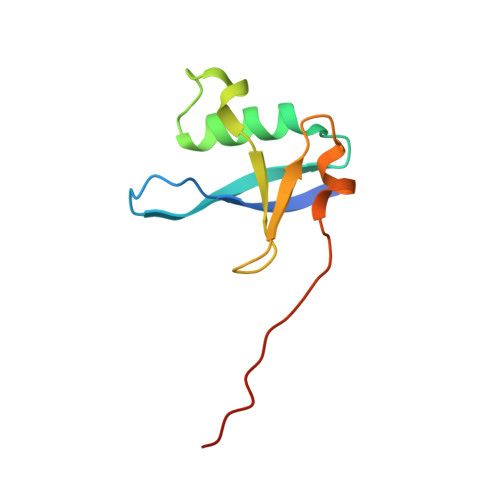
Structures of RGL1 RAS-Association Domain in Complex with KRAS and the Oncogenic G12V Mutant.
Eves, B.J., Gebregiworgis, T., Gasmi-Seabrook, G.M.C., Kuntz, D.A., Prive, G.G., Marshall, C.B., Ikura, M.(2022) J Mol Biology 434: 167527-167527
- PubMed: 35257782
- DOI: https://doi.org/10.1016/j.jmb.2022.167527
- Primary Citation of Related Structures:
7SCW, 7SCX - PubMed Abstract:
Ral Guanine Nucleotide Dissociation Stimulator Like 1 (RGL1) is a RAS effector protein that activates Ral GTPase by stimulating nucleotide exchange. Most structures of RAS-effector complexes are for the HRAS isoform; relatively few KRAS-effector structures have been solved, even though KRAS mutations are more frequent in human cancers. We determined crystal structures of KRAS/RGL1-RAS-association (RA) domain complexes and characterized the interaction in solution using nuclear magnetic resonance spectroscopy, size-exclusion chromatography combined with multi-angle light scattering and biolayer interferometry. We report structures of wild-type KRAS and the oncogenic G12V mutant in complex with the RA domain of RGL1 at < 2 Å resolution. KRAS WT /RGL1-RA crystallized as a 1:1 heterodimer, whilst KRAS G12V /RGL1-RA crystallized as a heterotetrameric structure in which RGL1-RA dimerized via domain-swapping the C-terminal beta-strand. Solution data indicated that KRAS WT and KRAS G12V in complex with RGL1-RA both exist predominantly as 1:1 dimers, while tetramerization occurs through very slow association. Through detailed structural analyses, the distance and angle between RAS α1 helix and RBD/RA α1 helix were found to differ significantly among RAS and RBD/RA complexes. The KRAS/RGL1-RA structures possess some of the largest α1 RAS /α1 Effector distances (21.7-22.2 Å), whereas the corresponding distances in previously reported RAS/RAF complexes are significantly shorter (15.2-17.7 Å). Contact map analysis identified unique structural signatures involving contacts between the β1-β2 loop of RA and the α1 helix of RAS, clearly distinguishing the KRAS/RGL1-RA (and other RAS/RA complexes) from RAS/RBD complexes. These results demonstrate that RAS effectors employ an assortment of finely-tuned docking surfaces to achieve optimal interactions with RAS.
- Princess Margaret Cancer Centre, University Health Network, Toronto, ON M5G 1L7, Canada. Electronic address: https://twitter.com/@B_Eves.
Organizational Affiliation: