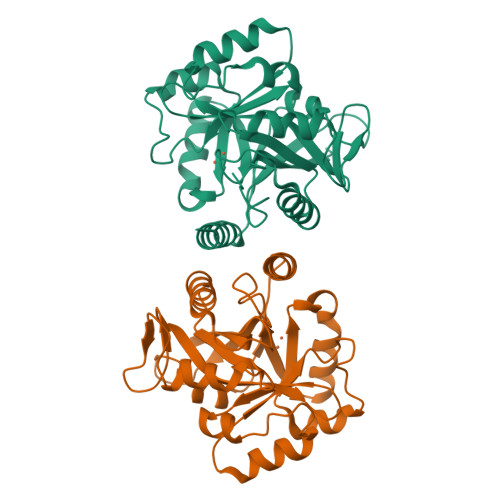
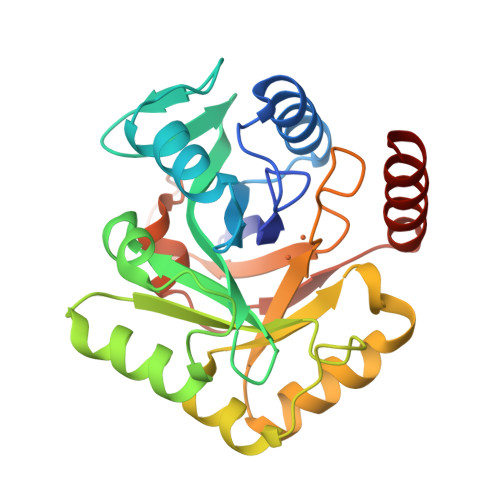
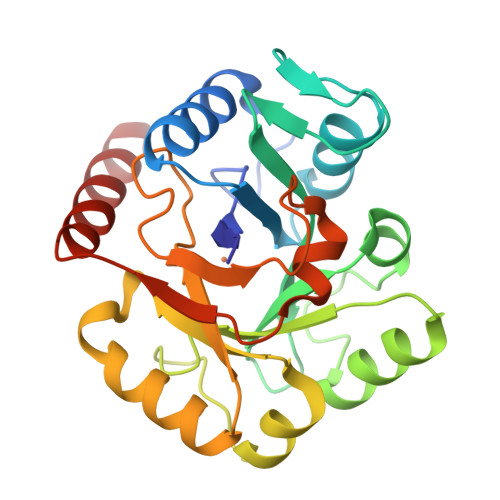
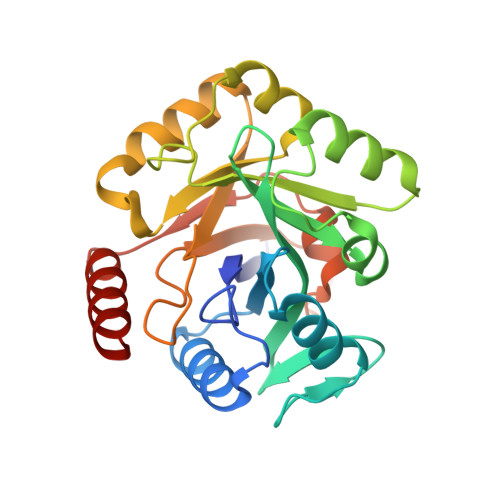
Biosynthesis of fosfomycin in pseudomonads reveals an unexpected enzymatic activity in the metallohydrolase superfamily.
Simon, M.A., Ongpipattanakul, C., Nair, S.K., van der Donk, W.A.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34074759
- DOI: https://doi.org/10.1073/pnas.2019863118
- Primary Citation of Related Structures:
7RTY - PubMed Abstract:
The epoxide-containing phosphonate natural product fosfomycin is a broad-spectrum antibiotic used in the treatment of cystitis. Fosfomycin is produced by both the plant pathogen Pseudomonas syringae and soil-dwelling streptomycetes. While the streptomycete pathway has recently been fully elucidated, the pseudomonad pathway is still mostly elusive. Through a systematic evaluation of heterologous expression of putative biosynthetic enzymes, we identified the central enzyme responsible for completing the biosynthetic pathway in pseudomonads. The missing transformation involves the oxidative decarboxylation of the intermediate 2-phosphonomethylmalate to a new intermediate, 3-oxo-4-phosphonobutanoate, by PsfC. Crystallographic studies reveal that PsfC unexpectedly belongs to a new class of diiron metalloenzymes that are part of the polymerase and histidinol phosphatase superfamily.
Organizational Affiliation:
Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801.