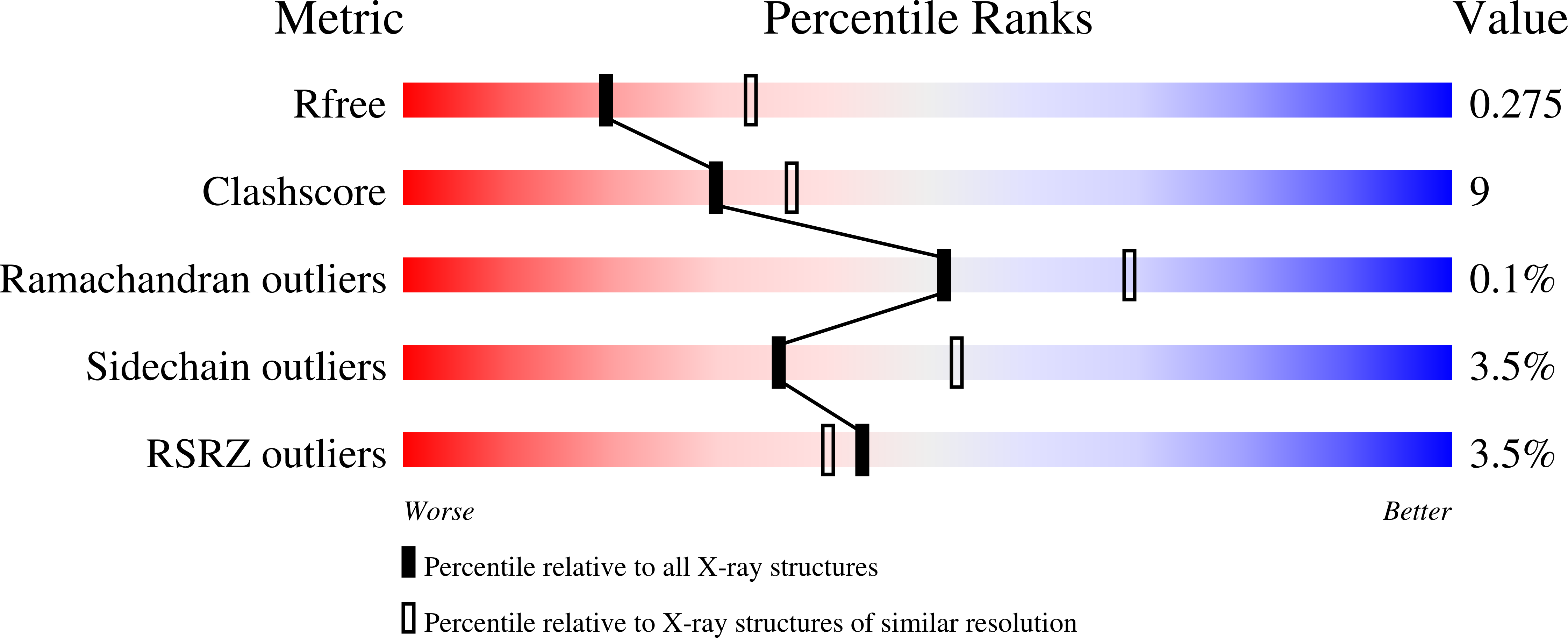
Energetic Basis and Design of Enzyme Function Demonstrated Using GFP, an Excited-State Enzyme.
Lin, C.Y., Romei, M.G., Mathews, I.I., Boxer, S.G.(2022) J Am Chem Soc 144: 3968-3978
- PubMed: 35200017
- DOI: https://doi.org/10.1021/jacs.1c12305
- Primary Citation of Related Structures:
7RRH, 7RRI, 7RRJ, 7RRK - PubMed Abstract:
The past decades have witnessed an explosion of de novo protein designs with a remarkable range of scaffolds. It remains challenging, however, to design catalytic functions that are competitive with naturally occurring counterparts as well as biomimetic or nonbiological catalysts. Although directed evolution often offers efficient solutions, the fitness landscape remains opaque. Green fluorescent protein (GFP), which has revolutionized biological imaging and assays, is one of the most redesigned proteins. While not an enzyme in the conventional sense, GFPs feature competing excited-state decay pathways with the same steric and electrostatic origins as conventional ground-state catalysts, and they exert exquisite control over multiple reaction outcomes through the same principles. Thus, GFP is an "excited-state enzyme". Herein we show that rationally designed mutants and hybrids that contain environmental mutations and substituted chromophores provide the basis for a quantitative model and prediction that describes the influence of sterics and electrostatics on excited-state catalysis of GFPs. As both perturbations can selectively bias photoisomerization pathways, GFPs with fluorescence quantum yields (FQYs) and photoswitching characteristics tailored for specific applications could be predicted and then demonstrated. The underlying energetic landscape, readily accessible via spectroscopy for GFPs, offers an important missing link in the design of protein function that is generalizable to catalyst design.
Organizational Affiliation:
Department of Chemistry, Stanford University, Stanford, California 94305, United States.