Design of a methotrexate-controlled chemical dimerization system and its use in bio-electronic devices.
Guo, Z., Smutok, O., Johnston, W.A., Walden, P., Ungerer, J.P.J., Peat, T.S., Newman, J., Parker, J., Nebl, T., Hepburn, C., Melman, A., Suderman, R.J., Katz, E., Alexandrov, K.(2021) Nat Commun 12: 7137-7137
- PubMed: 34880210
- DOI: https://doi.org/10.1038/s41467-021-27184-w
- Primary Citation of Related Structures:
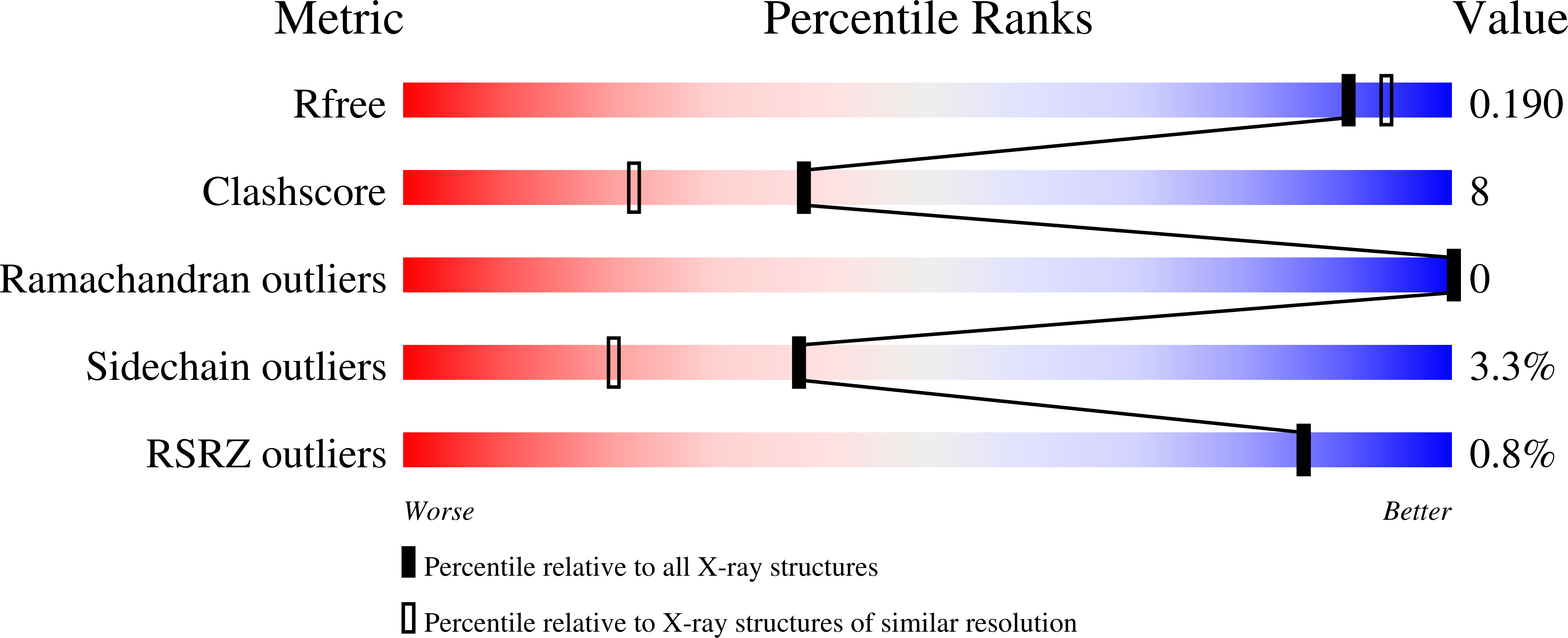
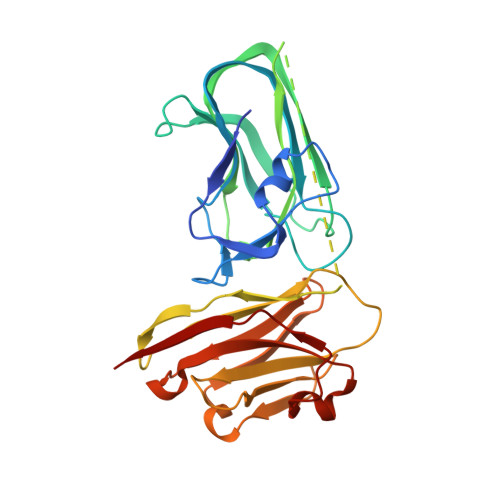
7RG7, 7RGA - PubMed Abstract:
Natural evolution produced polypeptides that selectively recognize chemical entities and their polymers, ranging from ions to proteins and nucleic acids. Such selective interactions serve as entry points to biological signaling and metabolic pathways. The ability to engineer artificial versions of such entry points is a key goal of synthetic biology, bioengineering and bioelectronics. We set out to map the optimal strategy for developing artificial small molecule:protein complexes that function as chemically induced dimerization (CID) systems. Using several starting points, we evolved CID systems controlled by a therapeutic drug methotrexate. Biophysical and structural analysis of methotrexate-controlled CID system reveals the critical role played by drug-induced conformational change in ligand-controlled protein complex assembly. We demonstrate utility of the developed CID by constructing electrochemical biosensors of methotrexate that enable quantification of methotrexate in human serum. Furthermore, using the methotrexate and functionally related biosensor of rapamycin we developed a multiplexed bioelectronic system that can perform repeated measurements of multiple analytes. The presented results open the door for construction of genetically encoded signaling systems for use in bioelectronics and diagnostics, as well as metabolic and signaling network engineering.
Organizational Affiliation:
ARC Centre of Excellence in Synthetic Biology, Sydney, NSW, Australia.