Identification and Characterization of an RRM-Containing, RNA Binding Protein in Acinetobacter baumannii .
Ciani, C., Perez-Rafols, A., Bonomo, I., Micaelli, M., Esposito, A., Zucal, C., Belli, R., D'Agostino, V.G., Bianconi, I., Calderone, V., Cerofolini, L., Massidda, O., Whalen, M.B., Fragai, M., Provenzani, A.(2022) Biomolecules 12
- PubMed: 35883478
- DOI: https://doi.org/10.3390/biom12070922
- Primary Citation of Related Structures:
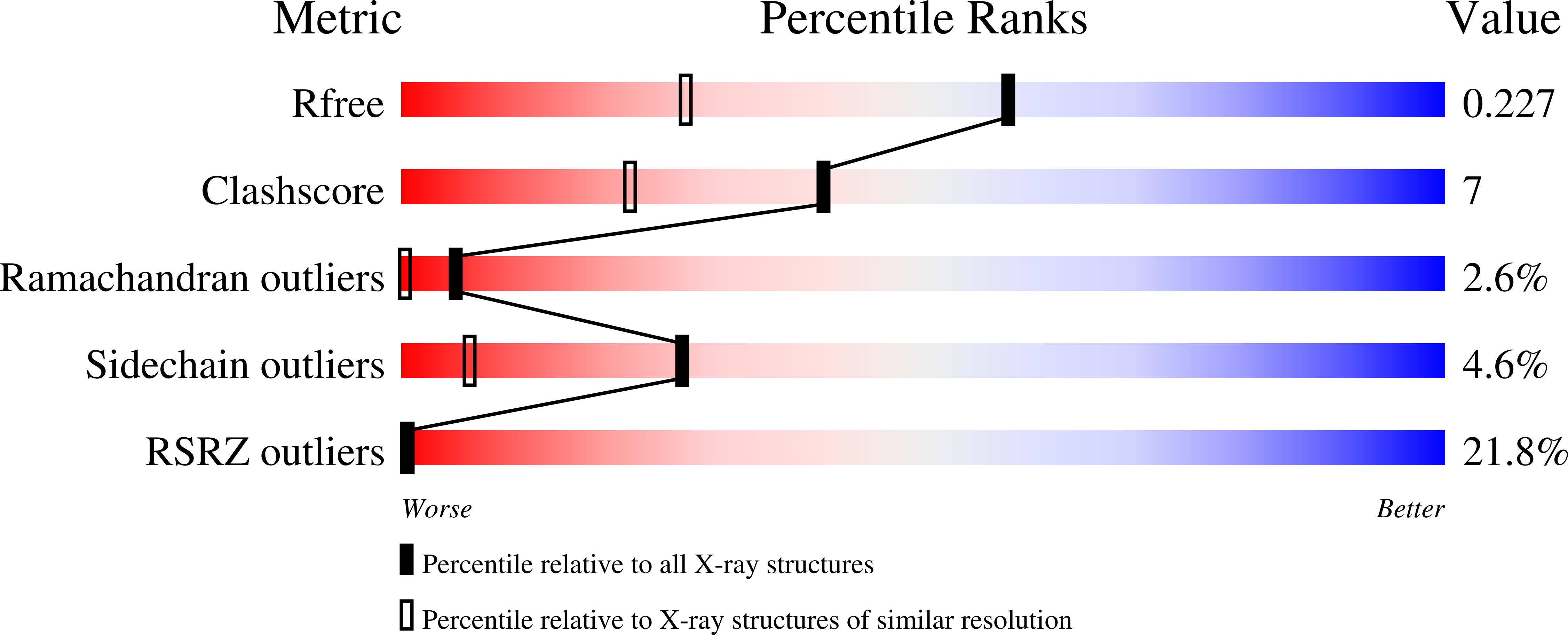
7QZP - PubMed Abstract:
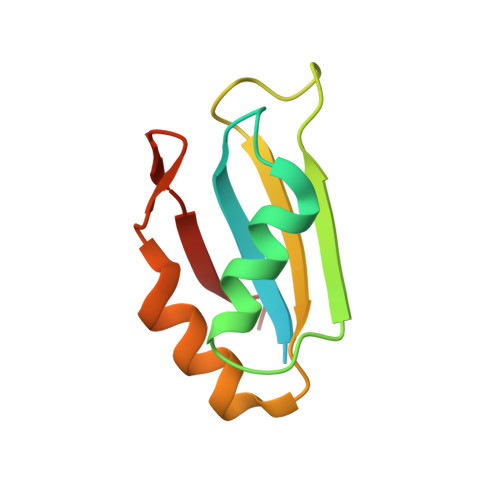
Acinetobacter baumannii is a Gram-negative pathogen, known to acquire resistance to antibiotics used in the clinic. The RNA-binding proteome of this bacterium is poorly characterized, in particular for what concerns the proteins containing RNA Recognition Motif (RRM). Here, we browsed the A. baumannii proteome for homologous proteins to the human HuR(ELAVL1), an RNA binding protein containing three RRMs. We identified a unique locus that we called AB - Elavl , coding for a protein with a single RRM with an average of 34% identity to the first HuR RRM. We also widen the research to the genomes of all the bacteria, finding 227 entries in 12 bacterial phyla. Notably we observed a partial evolutionary divergence between the RNP1 and RNP2 conserved regions present in the prokaryotes in comparison to the metazoan consensus sequence. We checked the expression at the transcript and protein level, cloned the gene and expressed the recombinant protein. The X-ray and NMR structural characterization of the recombinant AB-Elavl revealed that the protein maintained the typical β 1 α 1 β 2 β 3 α 2 β 4 and three-dimensional organization of eukaryotic RRMs. The biochemical analyses showed that, although the RNP1 and RNP2 show differences, it can bind to AU-rich regions like the human HuR, but with less specificity and lower affinity. Therefore, we identified an RRM-containing RNA-binding protein actually expressed in A. baumannii .
Organizational Affiliation:
Department of Cellular, Computational and Integrative Biology, DeCIBIO, Proteomics and MS Core Facility, University of Trento, 38123 Trento, Italy.