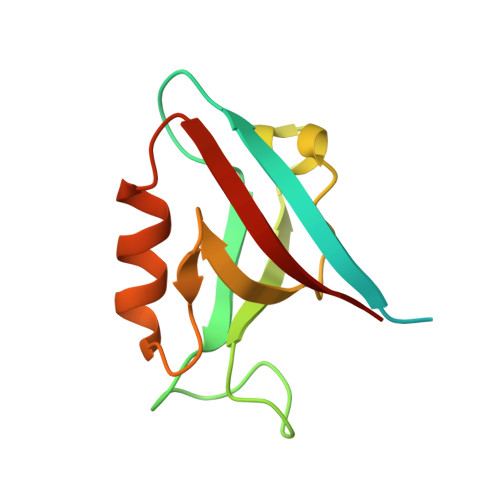
Molecular basis of Tick Born encephalitis virus NS5 mediated subversion of apico-basal cell polarity signalling.
Javorsky, A., Humbert, P.O., Kvansakul, M.(2022) Biochem J 479: 1303-1315
- PubMed: 35670457
- DOI: https://doi.org/10.1042/BCJ20220037
- Primary Citation of Related Structures:
7QS9, 7QSA, 7QSB - PubMed Abstract:
The Scribble (Scrib) protein is a conserved cell polarity regulator with anti-tumorigenic properties. Viruses like the Tick-born encephalitis virus (TBEV) target Scribble to establish a cellular environment supporting viral replication, which is ultimately associated with poor prognosis upon infection. The TBEV NS5 protein has been reported to harbour both an internal as well as a C-terminal PDZ binding motif (PBM), however only the internal PBM was shown to be an interactor with Scribble, with the interaction being mediated via the Scribble PDZ4 domain to antagonize host interferon responses. We examined the NS5 PBM motif interactions with all Scribble PDZ domains using isothermal titration calorimetry, which revealed that the proposed internal PBM did not interact with any Scribble PDZ domains. Instead, the C-terminal PBM of NS5 interacted with Scrib PDZ3. We then established the structural basis of these interactions by determining crystal structures of Scrib PDZ3 bound to the NS5 C-terminal PBM. Our findings provide a structural basis for Scribble PDZ domain and TBEV NS5 interactions and provide a platform to dissect the pathogenesis of TBEV and the role of cell polarity signalling using structure guided approaches.
- Department of Biochemistry & Chemistry, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia.
Organizational Affiliation: