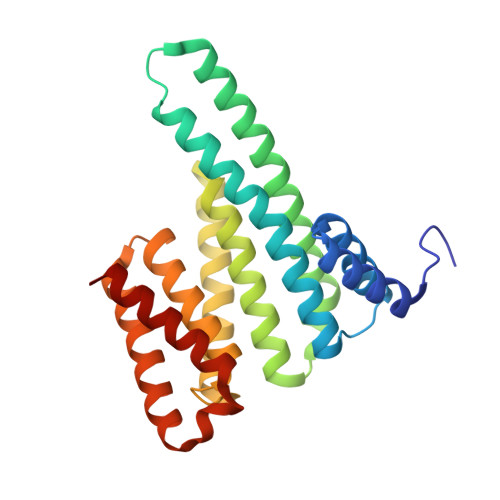
Macrocycle-stabilization of its interaction with 14-3-3 increases plasma membrane localization and activity of CFTR.
Stevers, L.M., Wolter, M., Carlile, G.W., Macdonald, D., Richard, L., Gielkens, F., Hanrahan, J.W., Thomas, D.Y., Chakka, S.K., Peterson, M.L., Thomas, H., Brunsveld, L., Ottmann, C.(2022) Nat Commun 13: 3586-3586
- PubMed: 35739107
- DOI: https://doi.org/10.1038/s41467-022-31206-6
- Primary Citation of Related Structures:
7QI1 - PubMed Abstract:
Impaired activity of the chloride channel CFTR is the cause of cystic fibrosis. 14-3-3 proteins have been shown to stabilize CFTR and increase its biogenesis and activity. Here, we report the identification and mechanism of action of a macrocycle stabilizing the 14-3-3/CFTR complex. This molecule rescues plasma membrane localization and chloride transport of F508del-CFTR and works additively with the CFTR pharmacological chaperone corrector lumacaftor (VX-809) and the triple combination Trikafta®. This macrocycle is a useful tool to study the CFTR/14-3-3 interaction and the potential of molecular glues in cystic fibrosis therapeutics.
- Laboratory of Chemical Biology, Department of Biomedical Engineering and Institute for Complex Molecular Systems, Eindhoven University of Technology, Eindhoven, The Netherlands.
Organizational Affiliation: