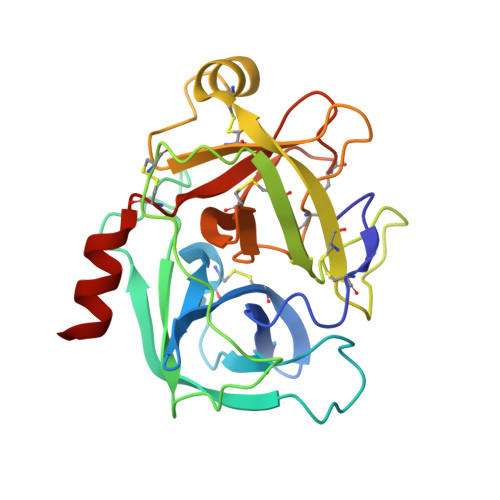
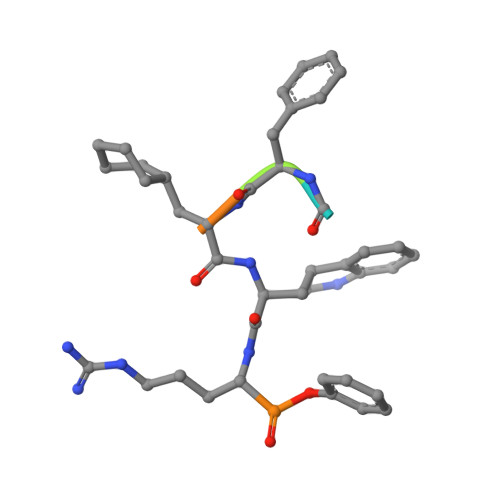
A KLK6 Activity-Based Probe Reveals a Role for KLK6 Activity in Pancreatic Cancer Cell Invasion.
Zhang, L., Lovell, S., De Vita, E., Jagtap, P.K.A., Lucy, D., Goya Grocin, A., Kjaer, S., Borg, A., Hennig, J., Miller, A.K., Tate, E.W.(2022) J Am Chem Soc 144: 22493-22504
- PubMed: 36413626
- DOI: https://doi.org/10.1021/jacs.2c07378
- Primary Citation of Related Structures:
7QFT, 7QFV - PubMed Abstract:
Pancreatic cancer has the lowest survival rate of all common cancers due to late diagnosis and limited treatment options. Serine hydrolases are known to mediate cancer progression and metastasis through initiation of signaling cascades and cleavage of extracellular matrix proteins, and the kallikrein-related peptidase (KLK) family of secreted serine proteases have emerging roles in pancreatic ductal adenocarcinoma (PDAC). However, the lack of reliable activity-based probes (ABPs) to profile KLK activity has hindered progress in validation of these enzymes as potential targets or biomarkers. Here, we developed potent and selective ABPs for KLK6 by using a positional scanning combinatorial substrate library and characterized their binding mode and interactions by X-ray crystallography. The optimized KLK6 probe IMP-2352 ( k obs / I = 11,000 M -1 s -1 ) enabled selective detection of KLK6 activity in a variety of PDAC cell lines, and we observed that KLK6 inhibition reduced the invasiveness of PDAC cells that secrete active KLK6. KLK6 inhibitors were combined with N-terminomics to identify potential secreted protein substrates of KLK6 in PDAC cells, providing insights into KLK6-mediated invasion pathways. These novel KLK6 ABPs offer a toolset to validate KLK6 and associated signaling partners as targets or biomarkers across a range of diseases.
- Department of Chemistry, Molecular Sciences Research Hub, Imperial College London, London W12 0BZ, U.K.
Organizational Affiliation: