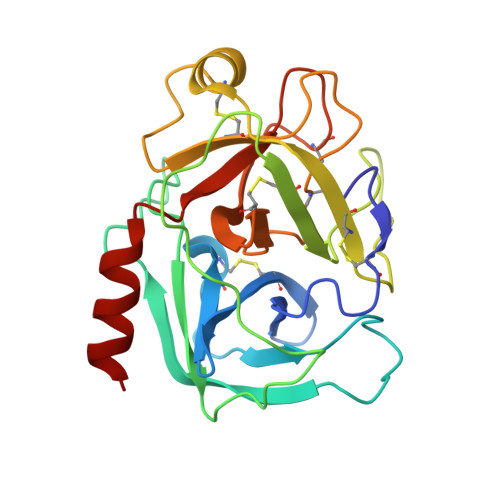
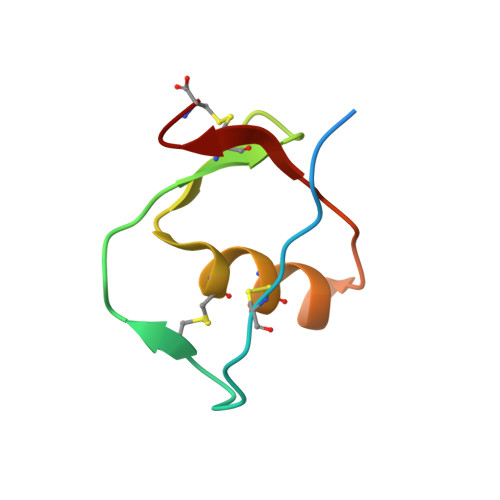
Structural and Biophysical Insights into SPINK1 Bound to Human Cationic Trypsin.
Nagel, F., Palm, G.J., Geist, N., McDonnell, T.C.R., Susemihl, A., Girbardt, B., Mayerle, J., Lerch, M.M., Lammers, M., Delcea, M.(2022) Int J Mol Sci 23
- PubMed: 35408828
- DOI: https://doi.org/10.3390/ijms23073468
- Primary Citation of Related Structures:
7QE8, 7QE9 - PubMed Abstract:
(1) The serine protease inhibitor Kazal type 1 (SPINK1) inhibits trypsin activity in zymogen granules of pancreatic acinar cells. Several mutations in the SPINK1 gene are associated with acute recurrent pancreatitis (ARP) and chronic pancreatitis (CP). The most common variant is SPINK1 p.N34S. Although this mutation was identified two decades ago, the mechanism of action has remained elusive. (2) SPINK1 and human cationic trypsin (TRY1) were expressed in E. coli , and inhibitory activities were determined. Crystals of SPINK1-TRY1 complexes were grown by using the hanging-drop method, and phases were solved by molecular replacement. (3) Both SPINK1 variants show similar inhibitory behavior toward TRY1. The crystal structures are almost identical, with minor differences in the mutated loop. Both complexes show an unexpected rotamer conformation of the His63 residue in TRY1, which is a member of the catalytic triad. (4) The SPINK1 p.N34S mutation does not affect the inhibitory behavior or the overall structure of the protein. Therefore, the pathophysiological mechanism of action of the p.N34S variant cannot be explained mechanistically or structurally at the protein level. The observed histidine conformation is part of a mechanism for SPINK1 that can explain the exceptional proteolytic stability of this inhibitor.
- Biophysical Chemistry, Institute of Biochemistry, University of Greifswald, 17489 Greifswald, Germany.
Organizational Affiliation: