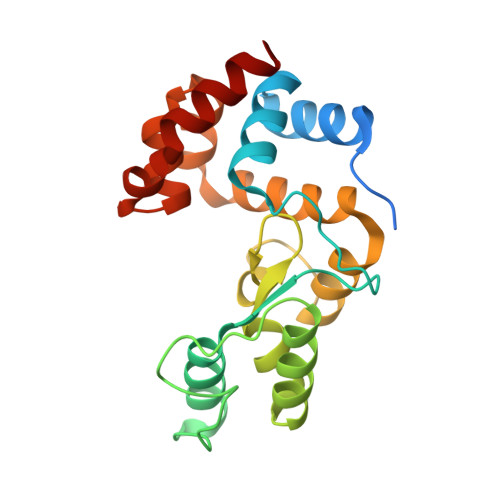
Monomodular Pseudomonas aeruginosa phage JG004 lysozyme (Pae87) contains a bacterial surface-active antimicrobial peptide-like region and a possible substrate-binding subdomain.
Vazquez, R., Seoane-Blanco, M., Rivero-Buceta, V., Ruiz, S., van Raaij, M.J., Garcia, P.(2022) Acta Crystallogr D Struct Biol 78: 435-454
- PubMed: 35362467
- DOI: https://doi.org/10.1107/S2059798322000936
- Primary Citation of Related Structures:
7Q4S, 7Q4T - PubMed Abstract:
Phage lysins are a source of novel antimicrobials to tackle the bacterial antibiotic-resistance crisis. The engineering of phage lysins is being explored as a game-changing technological strategy to introduce a more precise approach in the way in which antimicrobial therapy is applied. Such engineering efforts will benefit from a better understanding of lysin structure and function. In this work, the antimicrobial activity of the endolysin from Pseudomonas aeruginosa phage JG004, termed Pae87, has been characterized. This lysin had previously been identified as an antimicrobial agent candidate that is able to interact with the Gram-negative surface and disrupt it. Further evidence is provided here based on a structural and biochemical study. A high-resolution crystal structure of Pae87 complexed with a peptidoglycan fragment showed a separate substrate-binding region within the catalytic domain, 18 Å away from the catalytic site and located on the opposite side of the lysin molecule. This substrate-binding region was conserved among phylogenetically related lysins lacking an additional cell-wall-binding domain, but not among those containing such a module. Two glutamic acids were identified to be relevant for the peptidoglycan-degradation activity, although the antimicrobial activity of Pae87 was seemingly unrelated. In contrast, an antimicrobial peptide-like region within the Pae87 C-terminus, named P87, was found to be able to actively disturb the outer membrane and display antibacterial activity by itself. Therefore, an antimicrobial mechanism for Pae87 is proposed in which the P87 peptide plays the role of binding to the outer membrane and disrupting the cell-wall function, either with or without the participation of the catalytic activity of Pae87.
- Microbial and Plant Biotechnology Department, Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC), Madrid, Spain.
Organizational Affiliation: