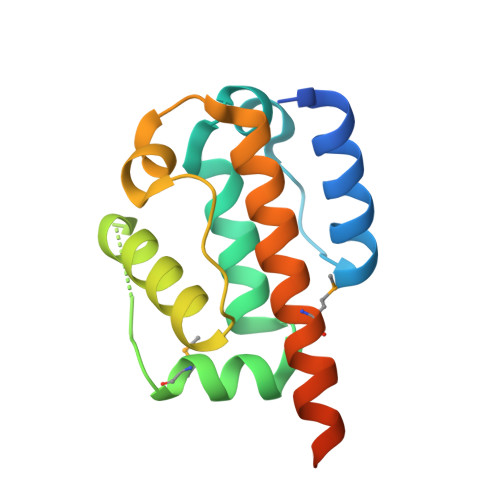
Structural validation and assessment of AlphaFold2 predictions for centrosomal and centriolar proteins and their complexes.
van Breugel, M., Rosa E Silva, I., Andreeva, A.(2022) Commun Biol 5: 312-312
- PubMed: 35383272
- DOI: https://doi.org/10.1038/s42003-022-03269-0
- Primary Citation of Related Structures:
7PT5, 7PTB - PubMed Abstract:
Obtaining the high-resolution structures of proteins and their complexes is a crucial aspect of understanding the mechanisms of life. Experimental structure determination methods are time-consuming, expensive and cannot keep pace with the growing number of protein sequences available through genomic DNA sequencing. Thus, the ability to accurately predict the structure of proteins from their sequence is a holy grail of structural and computational biology that would remove a bottleneck in our efforts to understand as well as rationally engineer living systems. Recent advances in protein structure prediction, in particular the breakthrough with the AI-based tool AlphaFold2 (AF2), hold promise for achieving this goal, but the practical utility of AF2 remains to be explored. Focusing on proteins with essential roles in centrosome and centriole biogenesis, we demonstrate the quality and usability of the AF2 prediction models and we show that they can provide important insights into the modular organization of two key players in this process, CEP192 and CEP44. Furthermore, we used the AF2 algorithm to elucidate and then experimentally validate previously unknown prime features in the structure of TTBK2 bound to CEP164, as well as the Chibby1-FAM92A complex for which no structural information was available to date. These findings have important implications in understanding the regulation and function of these complexes. Finally, we also discuss some practical limitations of AF2 and anticipate the implications for future research approaches in the centriole/centrosome field.
Organizational Affiliation:
Queen Mary University of London, School of Biological and Behavioural Sciences, 4 Newark Street, London, E1 2AT, UK. m.vanbreugel@qmul.ac.uk.