The Chemical Synthesis of Knob Domain Antibody Fragments.
Macpherson, A., Birtley, J.R., Broadbridge, R.J., Brady, K., Schulze, M.E.D., Tang, Y., Joyce, C., Saunders, K., Bogle, G., Horton, J., Kelm, S., Taylor, R.D., Franklin, R.J., Selby, M.D., Laabei, M., Wonfor, T., Hold, A., Stanley, P., Vadysirisack, D., Shi, J., van den Elsen, J., Lawson, A.D.G.(2021) ACS Chem Biol 16: 1757-1769
- PubMed: 34406751
- DOI: https://doi.org/10.1021/acschembio.1c00472
- Primary Citation of Related Structures:
7OP0 - PubMed Abstract:
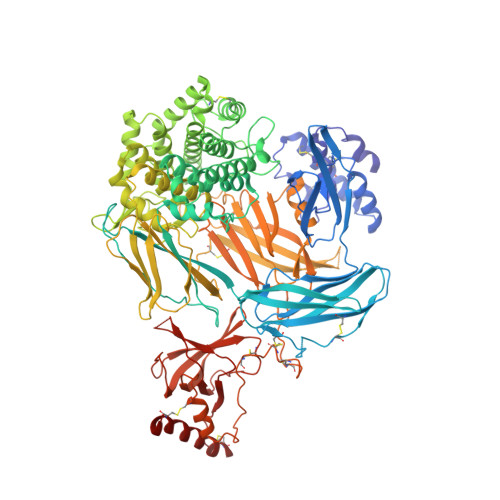
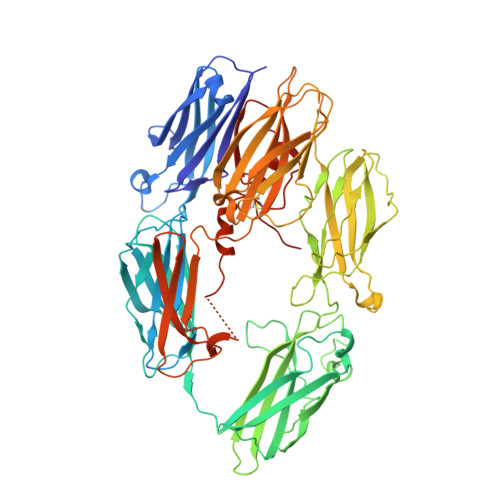
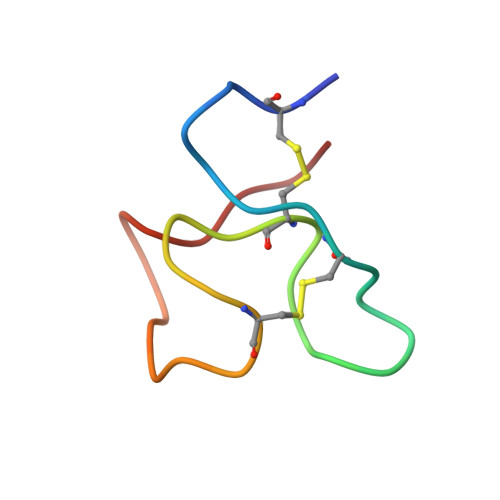
Cysteine-rich knob domains found in the ultralong complementarity determining regions of a subset of bovine antibodies are capable of functioning autonomously as 3-6 kDa peptides. While they can be expressed recombinantly in cellular systems, in this paper we show that knob domains are also readily amenable to a chemical synthesis, with a co-crystal structure of a chemically synthesized knob domain in complex with an antigen showing structural equivalence to the biological product. For drug discovery, following the immunization of cattle, knob domain peptides can be synthesized directly from antibody sequence data, combining the power and diversity of the bovine immune repertoire with the ability to rapidly incorporate nonbiological modifications. We demonstrate that, through rational design with non-natural amino acids, a paratope diversity can be massively expanded, in this case improving the efficacy of an allosteric peptide. As a potential route to further improve stability, we also performed head-to-tail cyclizations, exploiting the proximity of the N and C termini to synthesize functional, fully cyclic antibody fragments. Lastly, we highlight the stability of knob domains in plasma and, through pharmacokinetic studies, use palmitoylation as a route to extend the plasma half-life of knob domains in vivo. This study presents an antibody-derived medicinal chemistry platform, with protocols for solid-phase synthesis of knob domains, together with the characterization of their molecular structures, in vitro pharmacology, and pharmacokinetics.
- UCB Pharma, Slough SL1 3WE, U.K.
Organizational Affiliation: