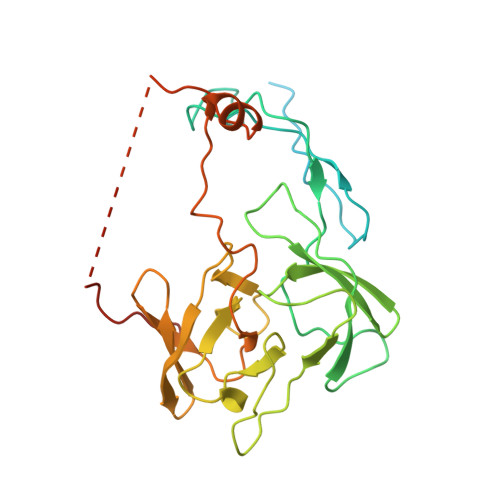
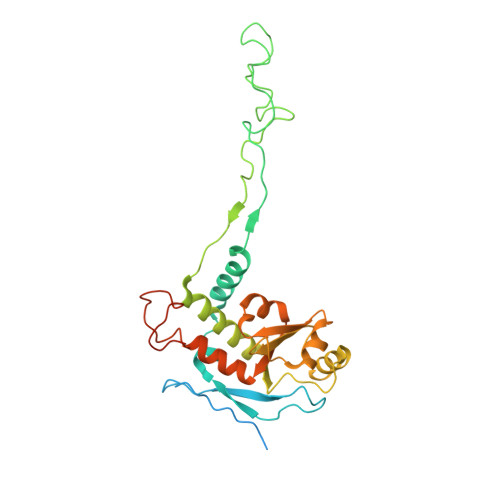
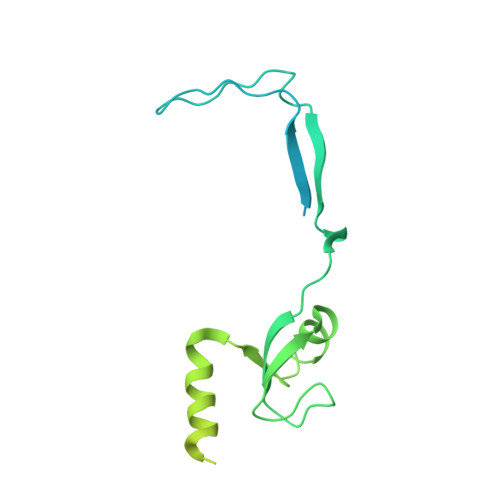
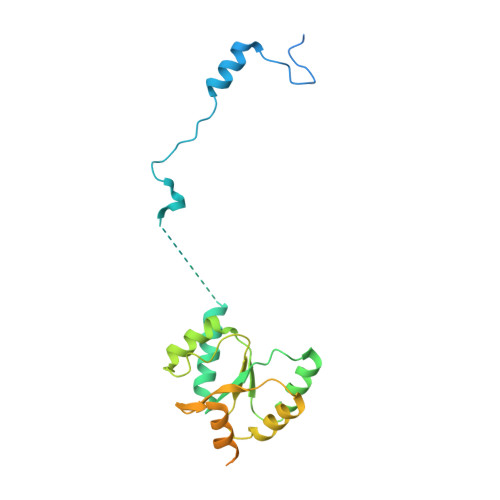
A distinct assembly pathway of the human 39S late pre-mitoribosome.
Cheng, J., Berninghausen, O., Beckmann, R.(2021) Nat Commun 12: 4544-4544
- PubMed: 34315873
- DOI: https://doi.org/10.1038/s41467-021-24818-x
- Primary Citation of Related Structures:
7OI6, 7OI7, 7OI8, 7OI9, 7OIA, 7OIB, 7OIC, 7OID, 7OIE - PubMed Abstract:
Assembly of the mitoribosome is largely enigmatic and involves numerous assembly factors. Little is known about their function and the architectural transitions of the pre-ribosomal intermediates. Here, we solve cryo-EM structures of the human 39S large subunit pre-ribosomes, representing five distinct late states. Besides the MALSU1 complex used as bait for affinity purification, we identify several assembly factors, including the DDX28 helicase, MRM3, GTPBP10 and the NSUN4-mTERF4 complex, all of which keep the 16S rRNA in immature conformations. The late transitions mainly involve rRNA domains IV and V, which form the central protuberance, the intersubunit side and the peptidyltransferase center of the 39S subunit. Unexpectedly, we find deacylated tRNA in the ribosomal E-site, suggesting a role in 39S assembly. Taken together, our study provides an architectural inventory of the distinct late assembly phase of the human 39S mitoribosome.
- Gene Center and Department for Biochemistry, LMU Munich, München, Germany. jcheng@genzentrum.lmu.de.
Organizational Affiliation: