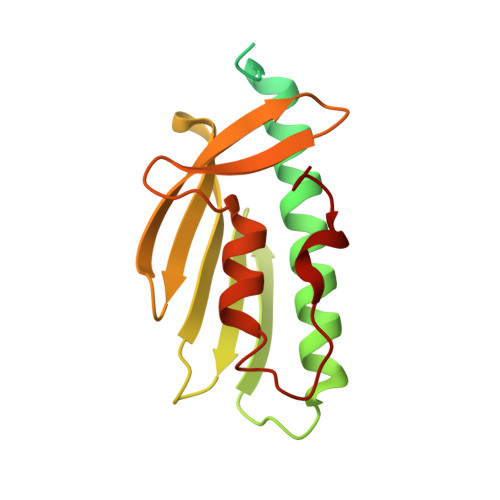
Drosophila melanogaster frataxin: protein crystal and predicted solution structure with identification of the iron-binding regions.
Rodrigues, A.V., Batelu, S., Hinton, T.V., Rotondo, J., Thompson, L., Brunzelle, J.S., Stemmler, T.L.(2023) Acta Crystallogr D Struct Biol 79: 22-30
- PubMed: 36601804
- DOI: https://doi.org/10.1107/S2059798322011639
- Primary Citation of Related Structures:
7N9I - PubMed Abstract:
Friedreich's ataxia (FRDA) is a hereditary cardiodegenerative and neurodegenerative disease that affects 1 in 50 000 Americans. FRDA arises from either a cellular inability to produce sufficient quantities or the production of a nonfunctional form of the protein frataxin, a key molecule associated with mitochondrial iron-sulfur cluster biosynthesis. Within the mitochondrial iron-sulfur cluster (ISC) assembly pathway, frataxin serves as an allosteric regulator for cysteine desulfurase, the enzyme that provides sulfur for [2Fe-2S] cluster assembly. Frataxin is a known iron-binding protein and is also linked to the delivery of ferrous ions to the scaffold protein, the ISC molecule responsible for the direct assembly of [2Fe-2S] clusters. The goal of this report is to provide structural details of the Drosophila melanogaster frataxin ortholog (Dfh), using both X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, in order to provide the foundational insight needed to understand the structure-function correlation of the protein. Additionally, NMR iron(II) titrations were used to provide metal contacts on the protein to better understand how it binds iron and aids its delivery to the ISC scaffold protein. Here, the structural and functional similarities of Dfh to its orthologs are also outlined. Structural data show that bacterial, yeast, human and Drosophila frataxins are structurally similar, apart from a structured C-terminus in Dfh that is likely to aid in protein stability. The iron-binding location on helix 1 and strand 1 of Dfh is also conserved across orthologs.
- Department of Biochemistry and Molecular Biology, Wayne State University, Detroit, Michigan, USA.
Organizational Affiliation: