Mechanism of LolCDE as a molecular extruder of bacterial triacylated lipoproteins
Sharma, S., Zhou, R., Wan, L., Feng, S., Song, K., Xu, C., Li, Y., Liao, M.(2021) Nat Commun 12: 4687
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lipoprotein transporter subunit LolE | A [auth B] | 414 | Escherichia coli | Mutation(s): 0 Gene Names: lolE Membrane Entity: Yes | 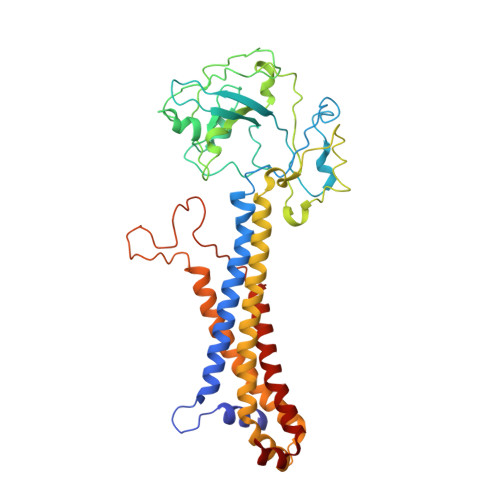 |
UniProt | |||||
Find proteins for P75958 (Escherichia coli (strain K12)) Explore P75958 Go to UniProtKB: P75958 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P75958 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lipo-releasing system transmembrane protein lolC | B [auth A] | 399 | Escherichia coli | Mutation(s): 0 EC: 3.6.3 Membrane Entity: Yes | 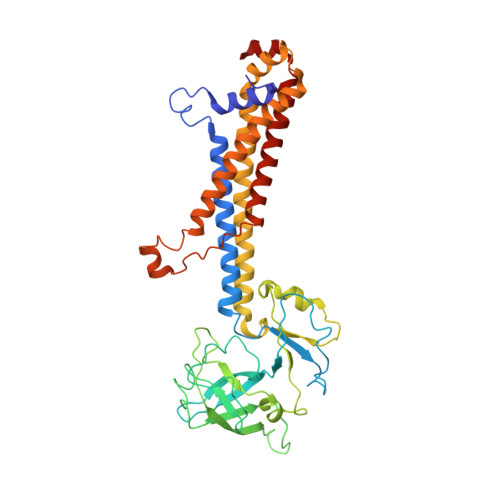 |
UniProt | |||||
Find proteins for P0ADC3 (Escherichia coli (strain K12)) Explore P0ADC3 Go to UniProtKB: P0ADC3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0ADC3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lipoprotein-releasing system ATP-binding protein LolD | C [auth D], D [auth C] | 236 | Escherichia coli | Mutation(s): 0 Gene Names: lolD_2, lolD, NCTC10767_05075, NCTC9001_00974 EC: 7.6.2 Membrane Entity: Yes | 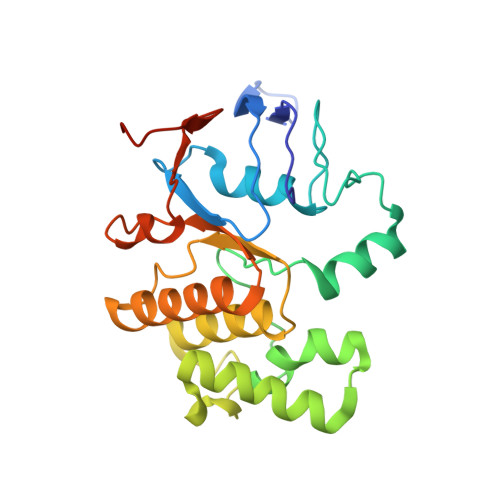 |
UniProt | |||||
Find proteins for P75957 (Escherichia coli (strain K12)) Explore P75957 Go to UniProtKB: P75957 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P75957 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| AOV Query on AOV | E [auth D], G [auth C] | ADP ORTHOVANADATE C10 H17 N5 O14 P2 V SWCHWRVRYDCWAN-AZGWGOJFSA-J |  | ||
| MG Query on MG | F [auth D], H [auth C] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | -- |