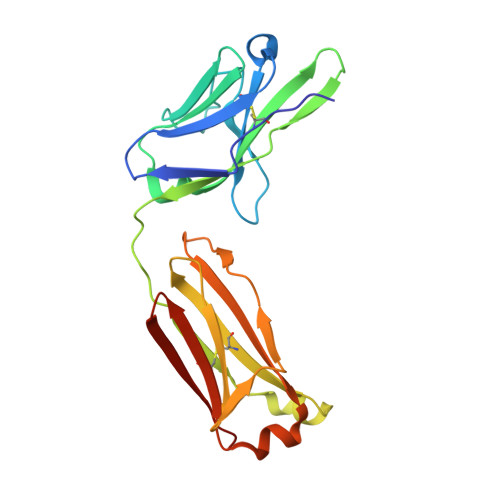
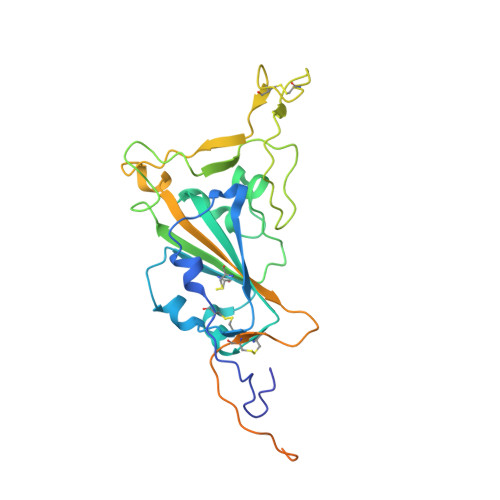
Isolation and characterization of cross-neutralizing coronavirus antibodies from COVID-19+ subjects.
Jennewein, M.F., MacCamy, A.J., Akins, N.R., Feng, J., Homad, L.J., Hurlburt, N.K., Seydoux, E., Wan, Y.H., Stuart, A.B., Edara, V.V., Floyd, K., Vanderheiden, A., Mascola, J.R., Doria-Rose, N., Wang, L., Yang, E.S., Chu, H.Y., Torres, J.L., Ozorowski, G., Ward, A.B., Whaley, R.E., Cohen, K.W., Pancera, M., McElrath, M.J., Englund, J.A., Finzi, A., Suthar, M.S., McGuire, A.T., Stamatatos, L.(2021) Cell Rep 36: 109353-109353
- PubMed: 34237283
- DOI: https://doi.org/10.1016/j.celrep.2021.109353
- Primary Citation of Related Structures:
7M3I - PubMed Abstract:
SARS-CoV-2 is one of three coronaviruses that have crossed the animal-to-human barrier and caused widespread disease in the past two decades. The development of a universal human coronavirus vaccine could prevent future pandemics. We characterize 198 antibodies isolated from four COVID-19+ subjects and identify 14 SARS-CoV-2 neutralizing antibodies. One targets the N-terminal domain (NTD), one recognizes an epitope in S2, and 11 bind the receptor-binding domain (RBD). Three anti-RBD neutralizing antibodies cross-neutralize SARS-CoV-1 by effectively blocking binding of both the SARS-CoV-1 and SARS-CoV-2 RBDs to the ACE2 receptor. Using the K18-hACE transgenic mouse model, we demonstrate that the neutralization potency and antibody epitope specificity regulates the in vivo protective potential of anti-SARS-CoV-2 antibodies. All four cross-neutralizing antibodies neutralize the B.1.351 mutant strain. Thus, our study reveals that epitopes in S2 can serve as blueprints for the design of immunogens capable of eliciting cross-neutralizing coronavirus antibodies.
- Fred Hutchinson Cancer Research Center, Vaccines and Infectious Disease Division, Seattle, WA 98109, USA.
Organizational Affiliation: