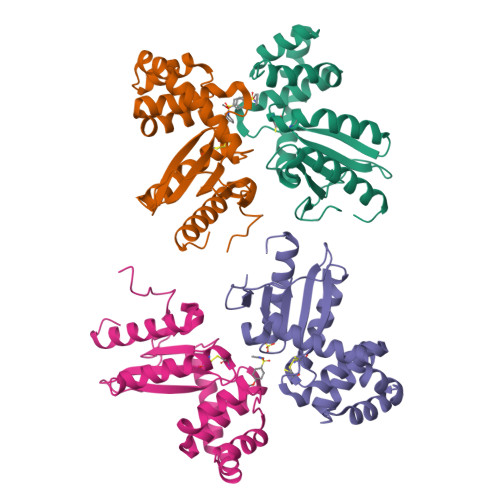
Identification and characterization of two drug-like fragments that bind to the same cryptic binding pocket of Burkholderia pseudomallei DsbA.
Petit, G.A., Mohanty, B., McMahon, R.M., Nebl, S., Hilko, D.H., Wilde, K.L., Scanlon, M.J., Martin, J.L., Halili, M.A.(2022) Acta Crystallogr D Struct Biol 78: 75-90
- PubMed: 34981764
- DOI: https://doi.org/10.1107/S2059798321011475
- Primary Citation of Related Structures:
7LUH, 7LUJ - PubMed Abstract:
Disulfide-bond-forming proteins (Dsbs) play a crucial role in the pathogenicity of many Gram-negative bacteria. Disulfide-bond-forming protein A (DsbA) catalyzes the formation of the disulfide bonds necessary for the activity and stability of multiple substrate proteins, including many virulence factors. Hence, DsbA is an attractive target for the development of new drugs to combat bacterial infections. Here, two fragments, bromophenoxy propanamide (1) and 4-methoxy-N-phenylbenzenesulfonamide (2), were identified that bind to DsbA from the pathogenic bacterium Burkholderia pseudomallei, the causative agent of melioidosis. The crystal structures of oxidized B. pseudomallei DsbA (termed BpsDsbA) co-crystallized with 1 or 2 show that both fragments bind to a hydrophobic pocket that is formed by a change in the side-chain orientation of Tyr110. This conformational change opens a `cryptic' pocket that is not evident in the apoprotein structure. This binding location was supported by 2D-NMR studies, which identified a chemical shift perturbation of the Tyr110 backbone amide resonance of more than 0.05 p.p.m. upon the addition of 2 mM fragment 1 and of more than 0.04 p.p.m. upon the addition of 1 mM fragment 2. Although binding was detected by both X-ray crystallography and NMR, the binding affinity (K d ) for both fragments was low (above 2 mM), suggesting weak interactions with BpsDsbA. This conclusion is also supported by the crystal structure models, which ascribe partial occupancy to the ligands in the cryptic binding pocket. Small fragments such as 1 and 2 are not expected to have a high energetic binding affinity due to their relatively small surface area and the few functional groups that are available for intermolecular interactions. However, their simplicity makes them ideal for functionalization and optimization. The identification of the binding sites of 1 and 2 to BpsDsbA could provide a starting point for the development of more potent novel antimicrobial compounds that target DsbA and bacterial virulence.
Organizational Affiliation:
Griffith Institute for Drug Discovery, Griffith University, Building N75, 46 Don Young Road, Nathan, QLD 4111, Australia.