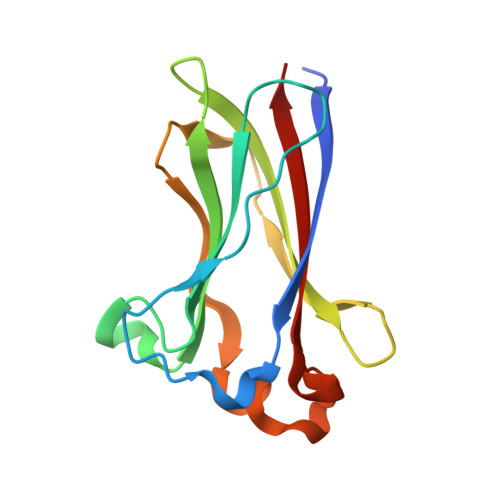
ATM-phosphorylated SPOP contributes to 53BP1 exclusion from chromatin during DNA replication.
Wang, D., Ma, J., Botuyan, M.V., Cui, G., Yan, Y., Ding, D., Zhou, Y., Krueger, E.W., Pei, J., Wu, X., Wang, L., Pei, H., McNiven, M.A., Ye, D., Mer, G., Huang, H.(2021) Sci Adv 7
- PubMed: 34144977
- DOI: https://doi.org/10.1126/sciadv.abd9208
- Primary Citation of Related Structures:
7LIN, 7LIO, 7LIP, 7LIQ - PubMed Abstract:
53BP1 activates nonhomologous end joining (NHEJ) and inhibits homologous recombination (HR) repair of DNA double-strand breaks (DSBs). Dissociation of 53BP1 from DSBs and consequent activation of HR, a less error-prone pathway than NHEJ, helps maintain genome integrity during DNA replication; however, the underlying mechanisms are not fully understood. Here, we demonstrate that E3 ubiquitin ligase SPOP promotes HR during S phase of the cell cycle by excluding 53BP1 from DSBs. In response to DNA damage, ATM kinase-catalyzed phosphorylation of SPOP causes a conformational change in SPOP, revealed by x-ray crystal structures, that stabilizes its interaction with 53BP1. 53BP1-bound SPOP induces polyubiquitination of 53BP1, eliciting 53BP1 extraction from chromatin by a valosin-containing protein/p97 segregase complex. Our work shows that SPOP facilitates HR repair over NHEJ during DNA replication by contributing to 53BP1 removal from chromatin. Cancer-derived SPOP mutations block SPOP interaction with 53BP1, inducing HR defects and chromosomal instability.
- Department of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA.
Organizational Affiliation: