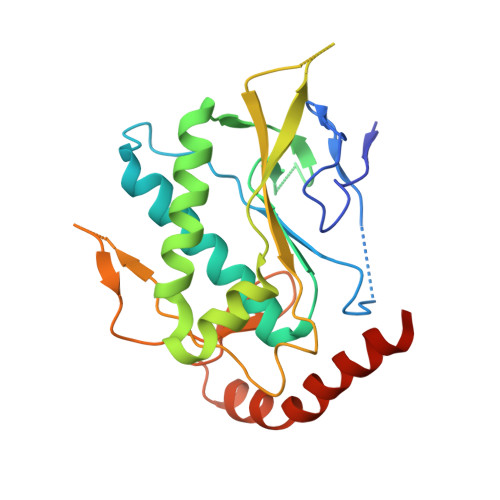
Structure and function of an effector domain in antiviral factors and tumor suppressors SAMD9 and SAMD9L.
Peng, S., Meng, X., Zhang, F., Pathak, P.K., Chaturvedi, J., Coronado, J., Morales, M., Mao, Y., Qian, S.B., Deng, J., Xiang, Y.(2022) Proc Natl Acad Sci U S A 119
- PubMed: 35046037
- DOI: https://doi.org/10.1073/pnas.2116550119
- Primary Citation of Related Structures:
7KSP - PubMed Abstract:
SAMD9 and SAMD9L (SAMD9/9L) are antiviral factors and tumor suppressors, playing a critical role in innate immune defense against poxviruses and the development of myeloid tumors. SAMD9/9L mutations with a gain-of-function (GoF) in inhibiting cell growth cause multisystem developmental disorders including many pediatric myelodysplastic syndromes. Predicted to be multidomain proteins with an architecture like that of the NOD-like receptors, SAMD9/9L molecular functions and domain structures are largely unknown. Here, we identified a SAMD9/9L effector domain that functions by binding to double-stranded nucleic acids (dsNA) and determined the crystal structure of the domain in complex with DNA. Aided with precise mutations that differentially perturb dsNA binding, we demonstrated that the antiviral and antiproliferative functions of the wild-type and GoF SAMD9/9L variants rely on dsNA binding by the effector domain. Furthermore, we showed that GoF variants inhibit global protein synthesis, reduce translation elongation, and induce proteotoxic stress response, which all require dsNA binding by the effector domain. The identification of the structure and function of a SAMD9/9L effector domain provides a therapeutic target for SAMD9/9L-associated human diseases.
- Department of Biochemistry and Molecular Biology, Oklahoma State University, Stillwater, OK 74078.
Organizational Affiliation: