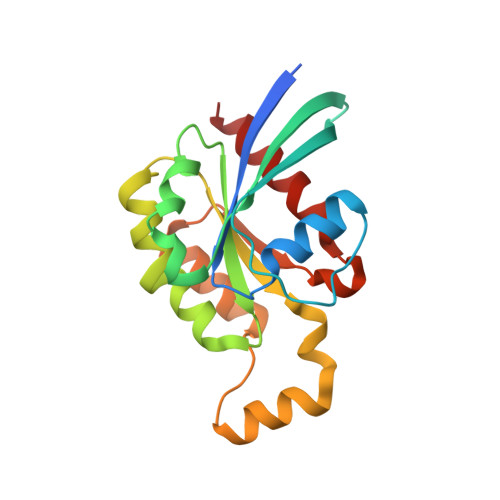
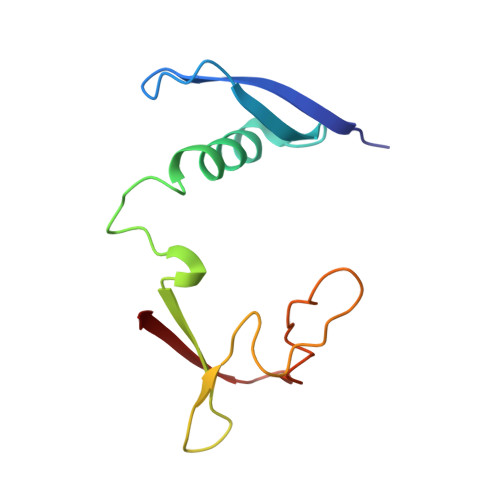
A putative structural mechanism underlying the antithetic effect of homologous RND1 and RhoD GTPases in mammalian plexin regulation.
Liu, Y., Ke, P., Kuo, Y.C., Wang, Y., Zhang, X., Song, C., Shan, Y.(2021) Elife 10
- PubMed: 34114565
- DOI: https://doi.org/10.7554/eLife.64304
- Primary Citation of Related Structures:
7KDC - PubMed Abstract:
Plexins are semaphorin receptors that play essential roles in mammalian neuronal axon guidance and in many other important mammalian biological processes. Plexin signaling depends on a semaphorin-induced dimerization mechanism and is modulated by small GTPases of the Rho family, of which RND1 serves as a plexin activator yet its close homolog RhoD an inhibitor. Using molecular dynamics (MD) simulations, we showed that RND1 reinforces the plexin dimerization interface, whereas RhoD destabilizes it due to their differential interaction with the cell membrane. Upon binding plexin at the Rho-GTPase-binding domain (RBD), RND1 and RhoD interact differently with the inner leaflet of the cell membrane and exert opposite effects on the dimerization interface via an allosteric network involving the RBD, RBD linkers, and a buttress segment adjacent to the dimerization interface. The differential membrane interaction is attributed to the fact that, unlike RND1, RhoD features a short C-terminal tail and a positively charged membrane interface.
- Center for Quantitative Biology, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China.
Organizational Affiliation: