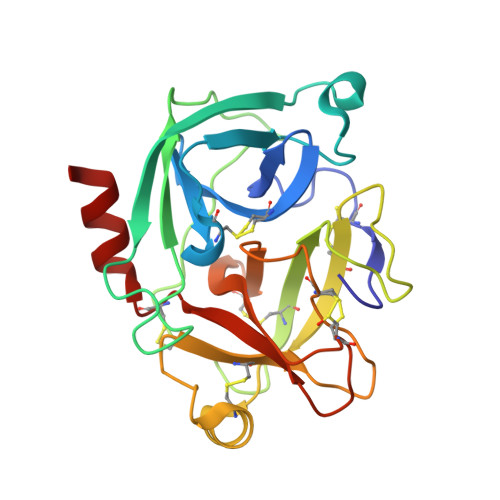
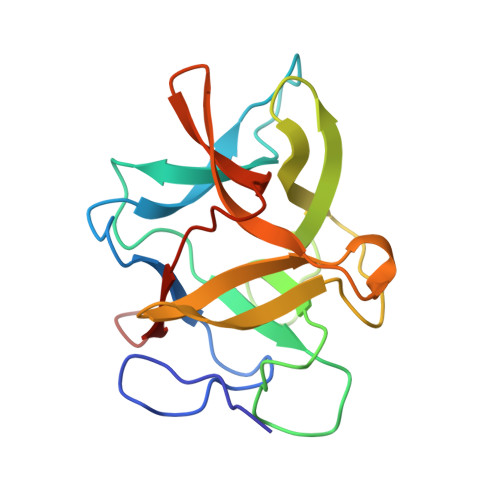
Structural studies of complexes of kallikrein 4 with wild-type and mutated forms of the Kunitz-type inhibitor BbKI.
Li, M., Srp, J., Mares, M., Wlodawer, A., Gustchina, A.(2021) Acta Crystallogr D Struct Biol 77: 1084-1098
- PubMed: 34342281
- DOI: https://doi.org/10.1107/S2059798321006483
- Primary Citation of Related Structures:
7JQK, 7JQN, 7JQO, 7JQV, 7JR1, 7JR2, 7JRX - PubMed Abstract:
Structures of BbKI, a recombinant Kunitz-type serine protease inhibitor from Bauhinia bauhinioides, complexed with human kallikrein 4 (KLK4) were determined at medium-to-high resolution in four crystal forms (space groups P3 1 21, P6 5 22, P2 1 and P6 1 ). Although the fold of the protein was virtually identical in all of the crystals, some significant differences were observed in the conformation of Arg64 of BbKI, the residue that occupies the S1 pocket in KLK4. Whereas this residue exhibited two orientations in the highest resolution structure (P3 1 21), making either a canonical trypsin-like interaction with Asp189 of KLK4 or an alternate interaction, only a single alternate orientation was observed in the other three structures. A neighboring disulfide, Cys191-Cys220, was partially or fully broken in all KLK4 structures. Four variants of BbKI in which Arg64 was replaced by Met, Phe, Ala and Asp were expressed and crystallized, and their structures were determined in complex with KLK4. Structures of the Phe and Met variants complexed with bovine trypsin and of the Phe variant complexed with α-chymotrypsin were also determined. Although the inhibitory potency of these variant forms of BbKI was lowered by up to four orders of magnitude, only small changes were seen in the vicinity of the mutated residues. Therefore, a totality of subtle differences in KLK4-BbKI interactions within the fully extended interface in the structures of these variants might be responsible for the observed effect. Screening of the BbKI variants against a panel of serine proteases revealed an altered pattern of inhibitory specificity, which was shifted towards that of chymotrypsin-like proteases for the hydrophobic Phe and Met P1 substitutions. This work reports the first structures of plant Kunitz inhibitors with S1-family serine proteases other than trypsin, as well as new insights into the specificity of inhibition of medically relevant kallikreins.
- Center for Structural Biology, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702, USA.
Organizational Affiliation: