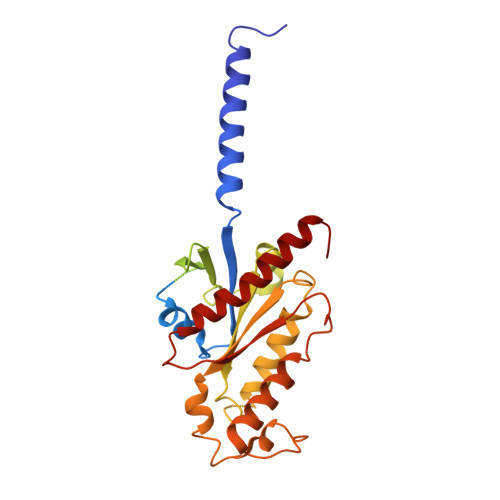
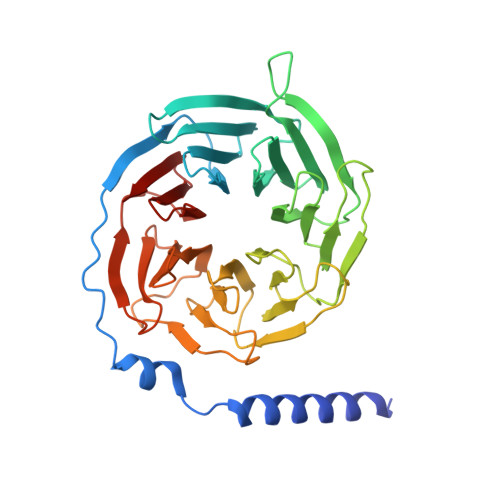
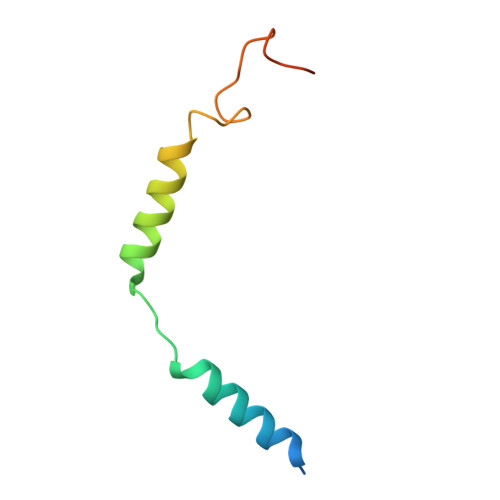
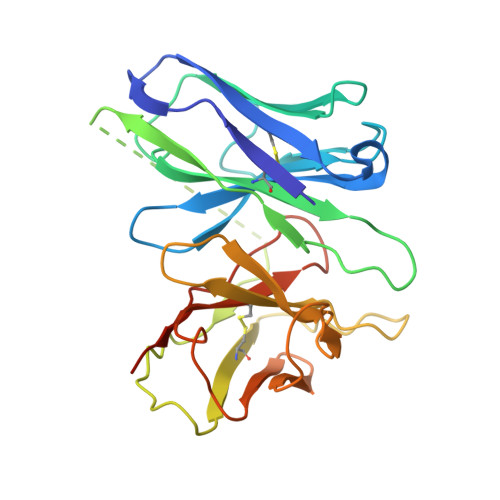
Structural basis for the constitutive activity and immunomodulatory properties of the Epstein-Barr virus-encoded G protein-coupled receptor BILF1.
Tsutsumi, N., Qu, Q., Mavri, M., Baggesen, M.S., Maeda, S., Waghray, D., Berg, C., Kobilka, B.K., Rosenkilde, M.M., Skiniotis, G., Garcia, K.C.(2021) Immunity 54: 1405-1416.e7
- PubMed: 34216564
- DOI: https://doi.org/10.1016/j.immuni.2021.06.001
- Primary Citation of Related Structures:
7JHJ - PubMed Abstract:
Epstein-Barr virus (EBV) encodes a G protein-coupled receptor (GPCR) termed BILF1 that is essential for EBV-mediated immunosuppression and oncogenesis. BILF1 couples with inhibitory G protein (Gi), the major intracellular signaling effector for human chemokine receptors, and exhibits constitutive signaling activity; the ligand(s) for BILF1 are unknown. We studied the origins of BILF1's constitutive activity through structure determination of BILF1 bound to the inhibitory G protein (Gi) heterotrimer. The 3.2-Å resolution cryo-electron microscopy structure revealed an extracellular loop within BILF1 that blocked the typical chemokine binding site, suggesting ligand-autonomous receptor activation. Rather, amino acid substitutions within BILF1 transmembrane regions at hallmark ligand-activated class A GPCR "microswitches" stabilized a constitutively active BILF1 conformation for Gi coupling in a ligand-independent fashion. Thus, the constitutive activity of BILF1 promotes immunosuppression and virulence independent of ligand availability, with implications for the function of GPCRs encoded by related viruses and for therapeutic targeting of EBV.
- Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA, USA; Department of Structural Biology, Stanford University School of Medicine, Stanford, CA, USA; Howard Hughes Medical Institute, Stanford University School of Medicine, Stanford, CA, USA.
Organizational Affiliation: