Crystal structure of human MICA mutants in complex with natural killer cell receptor NKG2D
Cai, W., Peng, S., Xu, T., Tian, Y., Li, Y., Liu, J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| NKG2-D type II integral membrane protein | 139 | Homo sapiens | Mutation(s): 0 Gene Names: KLRK1, D12S2489E, NKG2D | 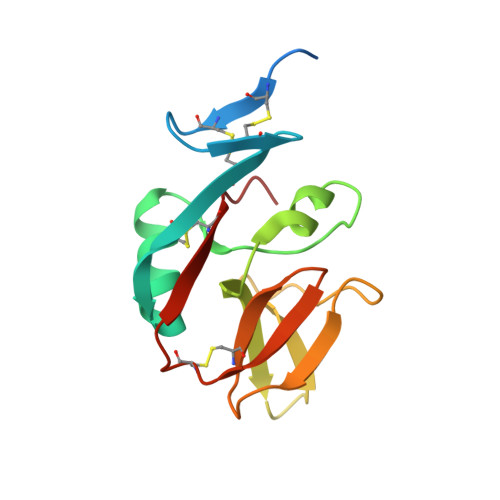 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P26718 (Homo sapiens) Explore P26718 Go to UniProtKB: P26718 | |||||
PHAROS: P26718 GTEx: ENSG00000213809 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P26718 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MHC class I polypeptide-related sequence A | 275 | Homo sapiens | Mutation(s): 5 Gene Names: MICA, PERB11.1 | 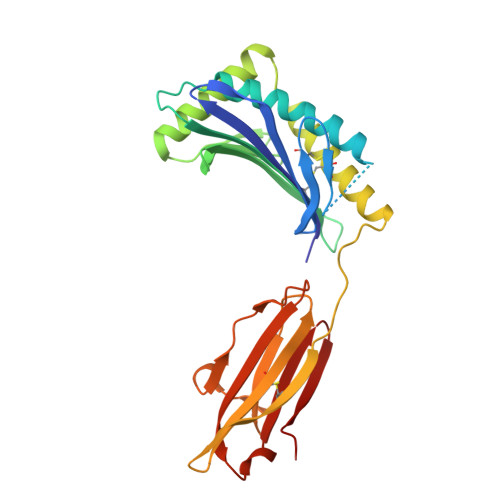 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q29983 (Homo sapiens) Explore Q29983 Go to UniProtKB: Q29983 | |||||
PHAROS: Q29983 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q29983 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | D [auth A], E [auth A], F [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 123.379 | α = 90 |
| b = 123.379 | β = 90 |
| c = 180.834 | γ = 90 |
| Software Name | Purpose |
|---|---|
| Aimless | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| MOLREP | phasing |