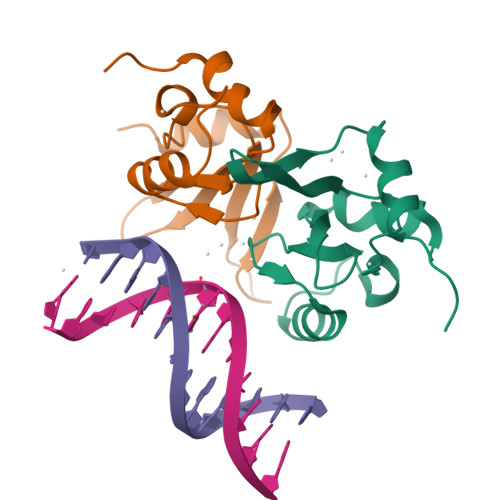
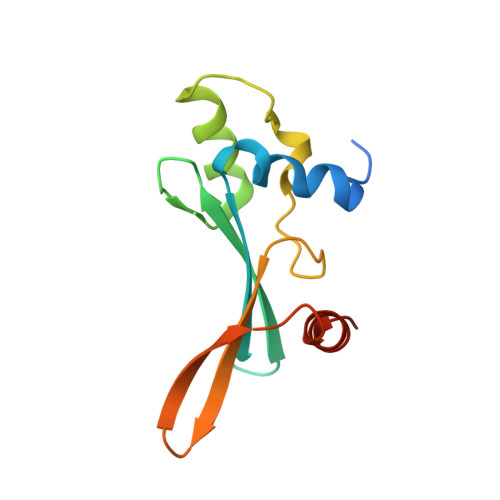
Structural basis of the TAM domain of BAZ2A in binding to DNA or RNA independent of methylation status.
Chen, S., Zhou, M., Dong, A., Loppnau, P., Wang, M., Min, J., Liu, K.(2021) J Biological Chem 297: 101351-101351
- PubMed: 34715126
- DOI: https://doi.org/10.1016/j.jbc.2021.101351
- Primary Citation of Related Structures:
7FHJ, 7MWI - PubMed Abstract:
Bromodomain adjacent to zinc finger domain protein 2A (BAZ2A) (also called transcription termination factor-1 interacting protein 5), a key component of the nucleolar remodeling complex, recruits the nucleolar remodeling complex to ribosomal RNA genes, leading to their transcriptional repression. In addition to its tandem plant homeodomain-bromodomain that is involved in binding to acetylated histone H4, BAZ2A also contains a methyl-CpG-binding domain (MBD)-like Tip5/ARBP/MBD (TAM) domain that shares sequence homology with the MBD. In contrast with the methyl-CpG-binding ability of the canonical MBD, the BAZ2A TAM domain has been shown to bind to promoter-associated RNAs of ribosomal RNA genes and promoter DNAs of other genes independent of DNA methylation. Nevertheless, how the TAM domain binds to RNA/DNA mechanistically remains elusive. Here, we characterized the DNA-/RNA-binding basis of the BAZ2A TAM domain by EMSAs, isothermal titration calorimetry binding assays, mutagenesis analysis, and X-ray crystallography. Our results showed that the TAM domain of BAZ2A selectively binds to dsDNA and dsRNA and that it binds to the backbone of dsDNA in a sequence nonspecific manner, which is distinct from the base-specific binding of the canonical MBD. Thus, our results explain why the TAM domain of BAZ2A does not specifically bind to mCG or TG dsDNA like the canonical MBD and also provide insights for further biological study of BAZ2A acting as a transcription factor in the future.
Organizational Affiliation:
Hubei Key Laboratory of Genetic Regulation and Integrative Biology, School of Life Sciences, Central China Normal University, Wuhan, PR China.