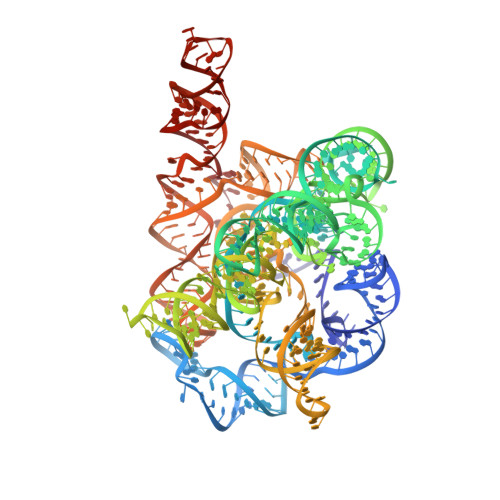
Cryo-EM structures of full-length Tetrahymena ribozyme at 3.1 angstrom resolution.
Su, Z., Zhang, K., Kappel, K., Li, S., Palo, M.Z., Pintilie, G.D., Rangan, R., Luo, B., Wei, Y., Das, R., Chiu, W.(2021) Nature 596: 603-607
- PubMed: 34381213
- DOI: https://doi.org/10.1038/s41586-021-03803-w
- Primary Citation of Related Structures:
7EZ0, 7EZ2 - PubMed Abstract:
Single-particle cryogenic electron microscopy (cryo-EM) has become a standard technique for determining protein structures at atomic resolution 1-3 . However, cryo-EM studies of protein-free RNA are in their early days. The Tetrahymena thermophila group I self-splicing intron was the first ribozyme to be discovered and has been a prominent model system for the study of RNA catalysis and structure-function relationships 4 , but its full structure remains unknown. Here we report cryo-EM structures of the full-length Tetrahymena ribozyme in substrate-free and bound states at a resolution of 3.1 Å. Newly resolved peripheral regions form two coaxially stacked helices; these are interconnected by two kissing loop pseudoknots that wrap around the catalytic core and include two previously unforeseen (to our knowledge) tertiary interactions. The global architecture is nearly identical in both states; only the internal guide sequence and guanosine binding site undergo a large conformational change and a localized shift, respectively, upon binding of RNA substrates. These results provide a long-sought structural view of a paradigmatic RNA enzyme and signal a new era for the cryo-EM-based study of structure-function relationships in ribozymes.
- The State Key Laboratory of Biotherapy and Cancer Center, Department of Geriatrics and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China. zsu@scu.edu.cn.
Organizational Affiliation: