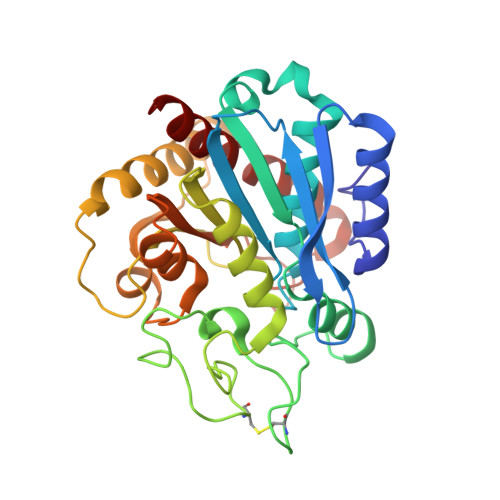
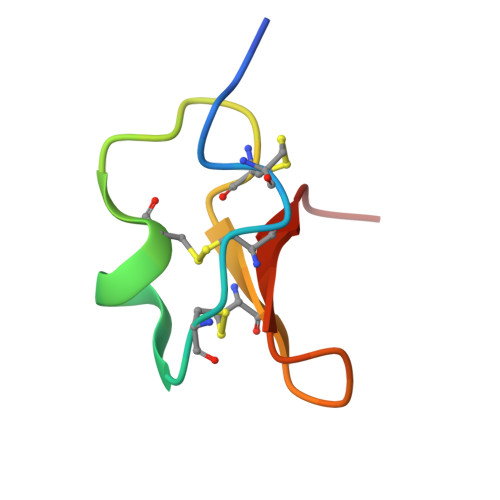
Structure of Aedes aegypti carboxypeptidase B1-inhibitor complex uncover the disparity between mosquito and non-mosquito insect carboxypeptidase inhibition mechanism.
Gavor, E., Choong, Y.K., Jobichen, C., Mok, Y.K., Kini, R.M., Sivaraman, J.(2021) Protein Sci 30: 2445-2456
- PubMed: 34658092
- DOI: https://doi.org/10.1002/pro.4212
- Primary Citation of Related Structures:
7EQZ - PubMed Abstract:
Metallocarboxypeptidases (MCPs) in the mosquito midgut play crucial roles in infection, as well as in mosquito dietary digestion, reproduction, and development. MCPs are also part of the digestive system of plant-feeding insects, representing key targets for inhibitor development against mosquitoes/mosquito-borne pathogens or as antifeedant molecules against plant-feeding insects. Notably, some non-mosquito insect B-type MCPs are primarily insensitive to plant protease inhibitors (PPIs) such as the potato carboxypeptidase inhibitor (PCI; MW 4 kDa), an inhibitor explored for cancer treatment and insecticide design. Here, we report the crystal structure of Aedes aegypti carboxypeptidase-B1 (CPBAe1)-PCI complex and compared the binding with that of PCI-insensitive CPBs. We show that PCI accommodation is determined by key differences in the active-site regions of MCPs. In particular, the loop regions α6-α7 (Leu 242 -Ser 250 ) and β8-α8 (Pro 269 -Pro 280 ) of CPBAe1 are replaced by α-helices in PCI-insensitive insect Helicoverpa zea CPBHz. These α-helices protrude into the active-site pocket of CPBHz, restricting PCI insertion and rendering the enzyme insensitive. We further compared our structure with the only other PCI complex available, bovine CPA1-PCI. The potency of PCI against CPBAe1 (K i = 14.7 nM) is marginally less than that of bovine CPA1 (K i = 5 nM). Structurally, the above loop regions that accommodate PCI binding in CPBAe1 are similar to that of bovine CPA1, although observed changes in proteases residues that interact with PCI could account for the differences in affinity. Our findings suggest that PCI sensitivity is largely dictated by structural interference, which broadens our understanding of carboxypeptidase inhibition as a mosquito population/parasite control strategy.
Organizational Affiliation:
Department of Biological Sciences, National University of Singapore, Singapore.