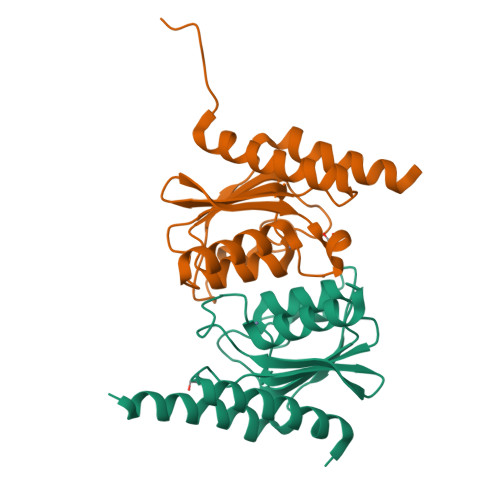
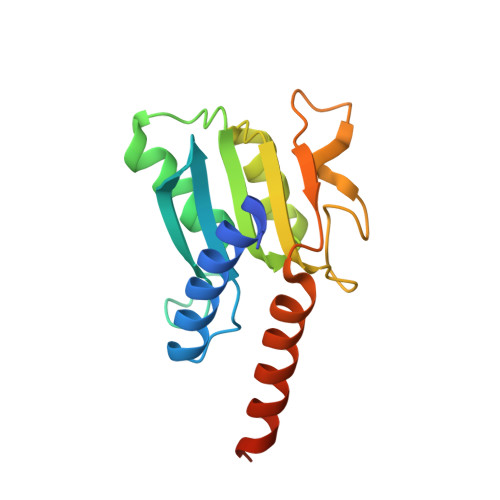
Deciphering protein microenvironment by using a cysteine specific switch-ON fluorescent probe.
Mariam, J., Hoskere Ashoka, A., Gaded, V., Ali, F., Malvi, H., Das, A., Anand, R.(2021) Org Biomol Chem 19: 5161-5168
- PubMed: 34037063
- DOI: https://doi.org/10.1039/d1ob00698c
- Primary Citation of Related Structures:
7CPH - PubMed Abstract:
Fluorescent probes provide an unparalleled opportunity to visualize and quantify dynamic events. Here, we employ a medium-size, cysteine specific coumarin based switch-ON fluorescent probe 'L' to track protein unfolding profiles and accessibility of cysteine residues in proteins. It was established that 'L' is highly selective and exhibits no artifact due to interaction with other bystander species. 'L' is able to gauge subtle changes in protein microenvironment and proved to be effective in delineating early unfolding events that are difficult to otherwise discern by classic techniques such as circular dichroism. By solving the X-ray structure of TadA and probing the temperature dependent fluorescence-ON response with native TadA and its cysteine mutants, it was revealed that unfolding occurs in a stage-wise manner and the regions that are functionally important form compact sub-domains and unfold at later stages. Our results assert that probe 'L' serves as an efficient tool to monitor subtle changes in protein structure and can be employed as a generic dye to study processes such as protein unfolding.
Organizational Affiliation:
Department of Chemistry, IIT Bombay, Mumbai-400076, India. ruchi@chem.iitb.ac.in.