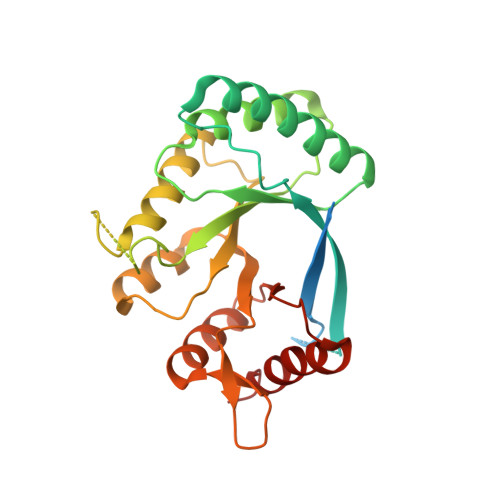
Biochemical Investigation of the Interaction of pICln, RioK1 and COPR5 with the PRMT5-MEP50 Complex.
Krzyzanowski, A., Gasper, R., Adihou, H., t Hart, P., Waldmann, H.(2021) Chembiochem 22: 1908-1914
- PubMed: 33624332
- DOI: https://doi.org/10.1002/cbic.202100079
- Primary Citation of Related Structures:
7BOC - PubMed Abstract:
The PRMT5-MEP50 methyltransferase complex plays a key role in various cancers and is regulated by different protein-protein interactions. Several proteins have been reported to act as adaptor proteins that recruit substrate proteins to the active site of PRMT5 for the methylation of arginine residues. To define the interaction between these adaptor proteins and PRMT5, we employed peptide truncation and mutation studies and prepared truncated protein constructs. We report the characterisation of the interface between the TIM barrel of PRMT5 and the adaptor proteins pICln, RioK1 and COPR5, and identify the consensus amino acid sequence GQF[D/E]DA[E/D] involved in binding. Protein crystallography revealed that the RioK1 derived peptide interacts with a novel PPI site.
- Department of Chemical Biology, Max Planck Institute of Molecular Physiology, Otto-Hahn-Strasse 11, 44227, Dortmund, Germany.
Organizational Affiliation: