Identification of Inhibitors of SARS-CoV-2 3CL-Pro Enzymatic Activity Using a Small Molecule in Vitro Repurposing Screen.
Kuzikov, M., Costanzi, E., Reinshagen, J., Esposito, F., Vangeel, L., Wolf, M., Ellinger, B., Claussen, C., Geisslinger, G., Corona, A., Iaconis, D., Talarico, C., Manelfi, C., Cannalire, R., Rossetti, G., Gossen, J., Albani, S., Musiani, F., Herzog, K., Ye, Y., Giabbai, B., Demitri, N., Jochmans, D., Jonghe, S., Rymenants, J., Summa, V., Tramontano, E., Beccari, A.R., Leyssen, P., Storici, P., Neyts, J., Gribbon, P., Zaliani, A.(2021) Acs Pharmacol Transl Sci 4: 1096-1110
- PubMed: 35287429
- DOI: https://doi.org/10.1021/acsptsci.0c00216
- Primary Citation of Related Structures:
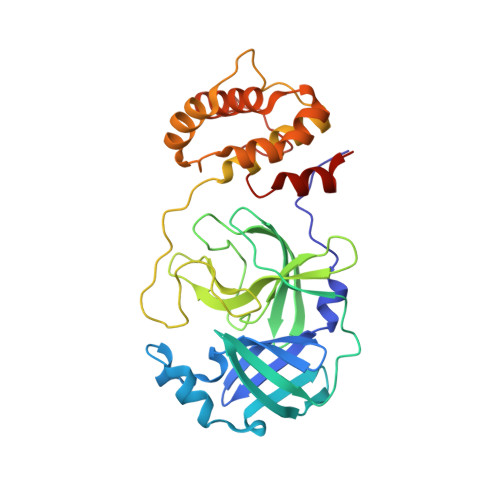
7B3E - PubMed Abstract:
Compound repurposing is an important strategy for the identification of effective treatment options against SARS-CoV-2 infection and COVID-19 disease. In this regard, SARS-CoV-2 main protease (3CL-Pro), also termed M-Pro, is an attractive drug target as it plays a central role in viral replication by processing the viral polyproteins pp1a and pp1ab at multiple distinct cleavage sites. We here report the results of a repurposing program involving 8.7 K compounds containing marketed drugs, clinical and preclinical candidates, and small molecules regarded as safe in humans. We confirmed previously reported inhibitors of 3CL-Pro and have identified 62 additional compounds with IC 50 values below 1 μM and profiled their selectivity toward chymotrypsin and 3CL-Pro from the Middle East respiratory syndrome virus. A subset of eight inhibitors showed anticytopathic effect in a Vero-E6 cell line, and the compounds thioguanosine and MG-132 were analyzed for their predicted binding characteristics to SARS-CoV-2 3CL-Pro. The X-ray crystal structure of the complex of myricetin and SARS-Cov-2 3CL-Pro was solved at a resolution of 1.77 Å, showing that myricetin is covalently bound to the catalytic Cys145 and therefore inhibiting its enzymatic activity.
- Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP), Schnackenburgallee 114, 22525 Hamburg, Germany.
Organizational Affiliation: