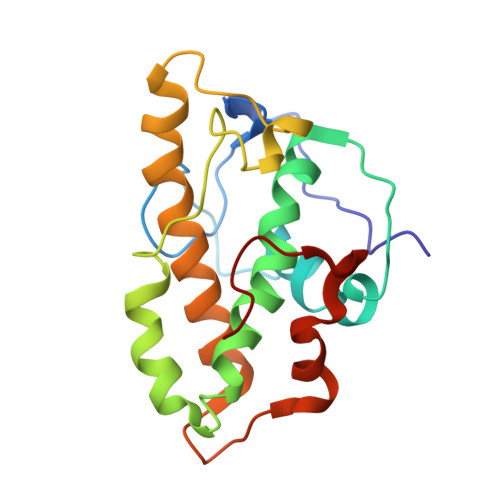
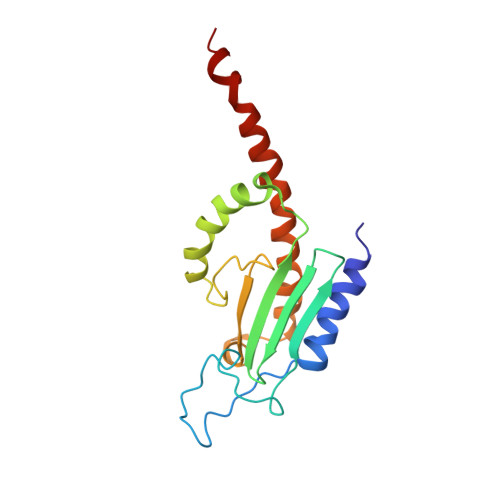
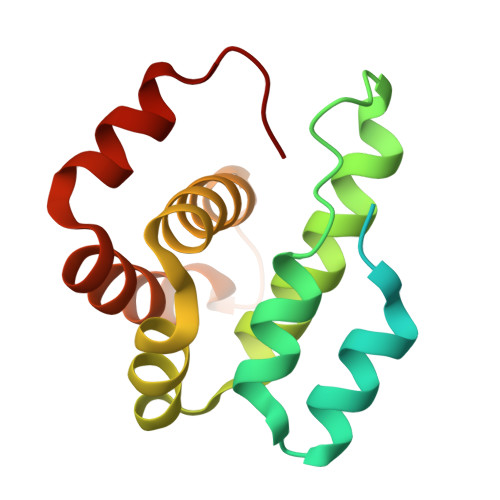
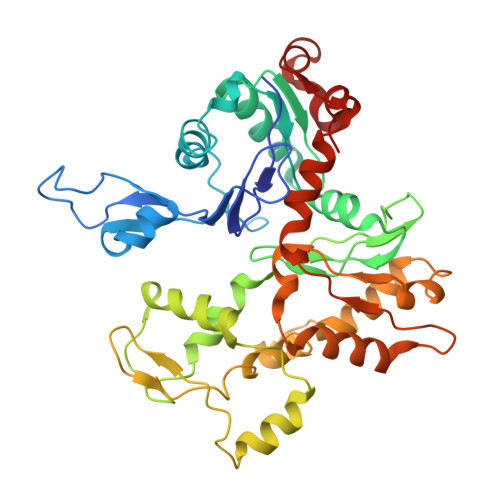
Cryo-electron tomography structure of Arp2/3 complex in cells reveals new insights into the branch junction.
Fassler, F., Dimchev, G., Hodirnau, V.V., Wan, W., Schur, F.K.M.(2020) Nat Commun 11: 6437-6437
- PubMed: 33353942
- DOI: https://doi.org/10.1038/s41467-020-20286-x
- Primary Citation of Related Structures:
7AQK - PubMed Abstract:
The actin-related protein (Arp)2/3 complex nucleates branched actin filament networks pivotal for cell migration, endocytosis and pathogen infection. Its activation is tightly regulated and involves complex structural rearrangements and actin filament binding, which are yet to be understood. Here, we report a 9.0 Å resolution structure of the actin filament Arp2/3 complex branch junction in cells using cryo-electron tomography and subtomogram averaging. This allows us to generate an accurate model of the active Arp2/3 complex in the branch junction and its interaction with actin filaments. Notably, our model reveals a previously undescribed set of interactions of the Arp2/3 complex with the mother filament, significantly different to the previous branch junction model. Our structure also indicates a central role for the ArpC3 subunit in stabilizing the active conformation.
- Institute of Science and Technology (IST) Austria, Klosterneuburg, Austria.
Organizational Affiliation: