Crystal structure of Shank1 PDZ domain with ARAP3-derived peptide
Mariam McAuley, M., Ali, M., Ivarsson, Y., Knapp, S., Joerger, A.C., Structural Genomics Consortium (SGC)To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SH3 and multiple ankyrin repeat domains protein 1,Arf-GAP with Rho-GAP domain, ANK repeat and PH domain-containing protein 3 | 140 | Rattus norvegicus, Homo sapiens This entity is chimeric | Mutation(s): 0 Gene Names: Shank1, ARAP3, CENTD3 | 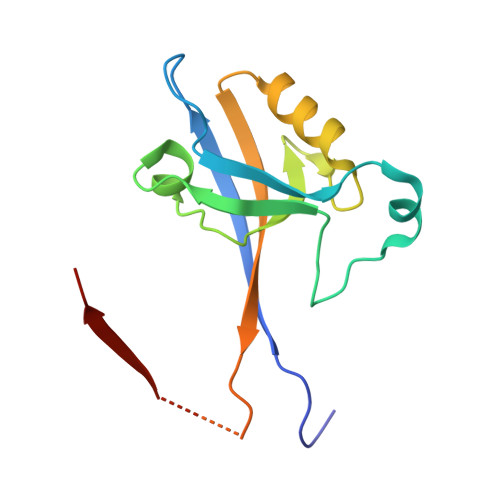 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8WWN8 (Homo sapiens) Explore Q8WWN8 Go to UniProtKB: Q8WWN8 | |||||
PHAROS: Q8WWN8 GTEx: ENSG00000120318 | |||||
Find proteins for Q9WV48 (Rattus norvegicus) Explore Q9WV48 Go to UniProtKB: Q9WV48 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | Q8WWN8Q9WV48 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EDO Query on EDO | C [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 67.799 | α = 90 |
| b = 67.799 | β = 90 |
| c = 248.967 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| H2020 Marie Curie Actions of the European Commission | 675341 |