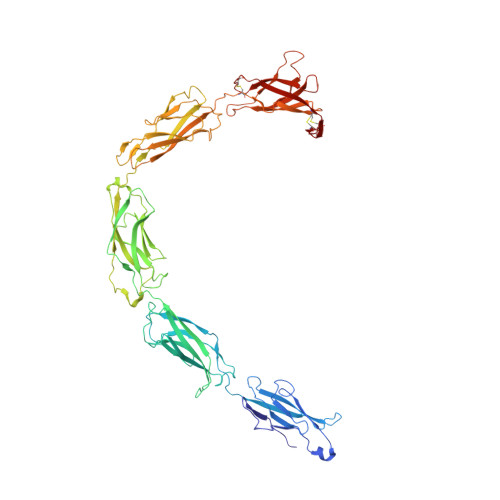
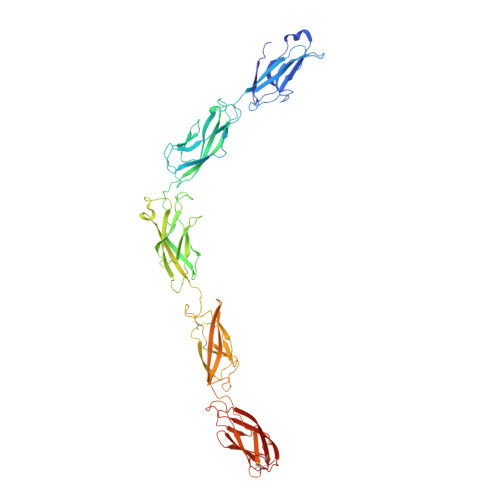Desmosome architecture derived from molecular dynamics simulations and cryo-electron tomography.
Sikora, M., Ermel, U.H., Seybold, A., Kunz, M., Calloni, G., Reitz, J., Vabulas, R.M., Hummer, G., Frangakis, A.S.(2020) Proc Natl Acad Sci U S A 117: 27132-27140
- PubMed: 33067392
- DOI: https://doi.org/10.1073/pnas.2004563117
- Primary Citation of Related Structures:
7A7D - PubMed Abstract:
Desmosomes are cell-cell junctions that link tissue cells experiencing intense mechanical stress. Although the structure of the desmosomal cadherins is known, the desmosome architecture-which is essential for mediating numerous functions-remains elusive. Here, we recorded cryo-electron tomograms (cryo-ET) in which individual cadherins can be discerned; they appear variable in shape, spacing, and tilt with respect to the membrane. The resulting sub-tomogram average reaches a resolution of ∼26 Å, limited by the inherent flexibility of desmosomes. To address this challenge typical of dynamic biological assemblies, we combine sub-tomogram averaging with atomistic molecular dynamics (MD) simulations. We generate models of possible cadherin arrangements and perform an in silico screening according to biophysical and structural properties extracted from MD simulation trajectories. We find a truss-like arrangement of cadherins that resembles the characteristic footprint seen in the electron micrograph. The resulting model of the desmosomal architecture explains their unique biophysical properties and strength.
- Theoretical Biophysics Department, Max Planck Institute for Biophysics, 60438 Frankfurt, Germany.
Organizational Affiliation: