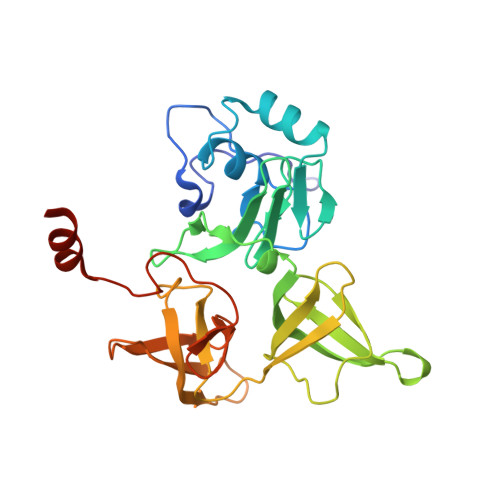
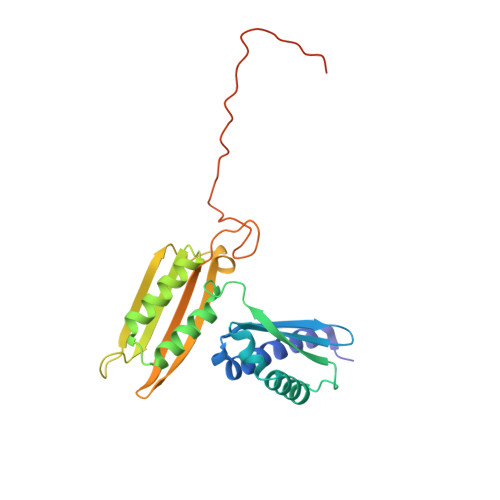
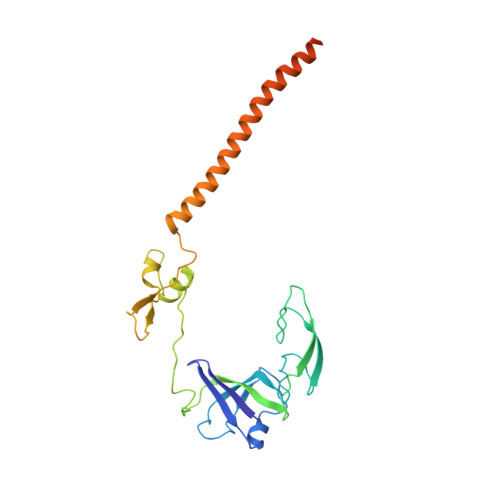
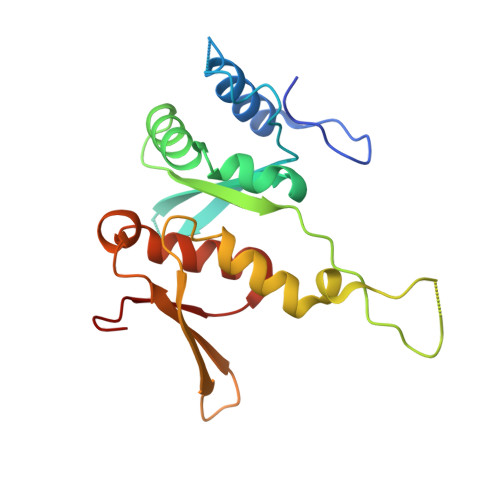
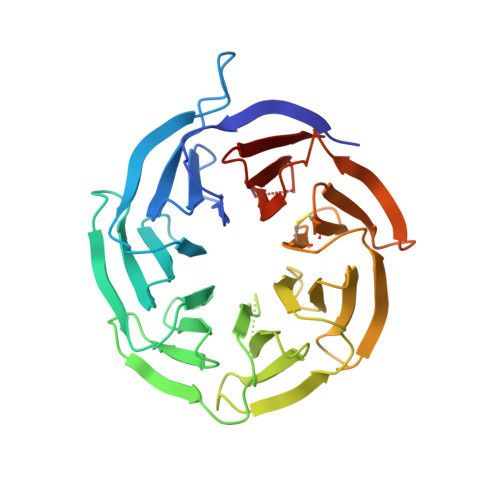
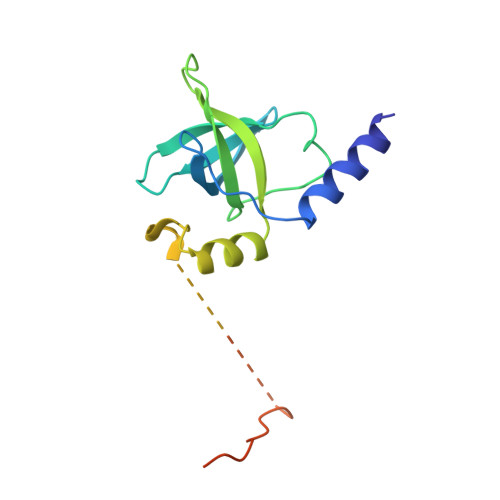
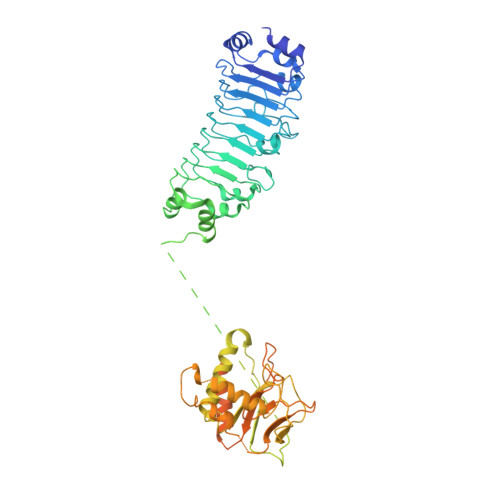
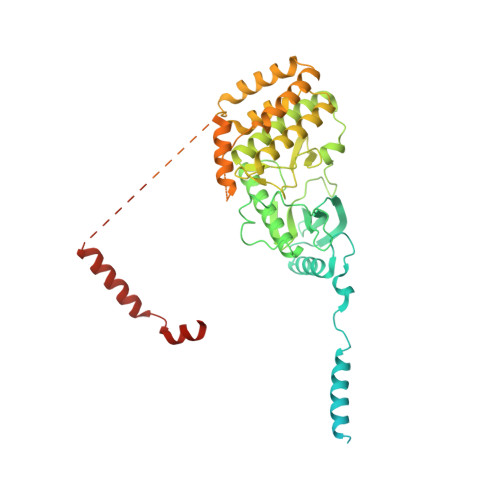
Structural basis for the final steps of human 40S ribosome maturation.
Ameismeier, M., Zemp, I., van den Heuvel, J., Thoms, M., Berninghausen, O., Kutay, U., Beckmann, R.(2020) Nature 587: 683-687
- PubMed: 33208940
- DOI: https://doi.org/10.1038/s41586-020-2929-x
- Primary Citation of Related Structures:
6ZXD, 6ZXE, 6ZXF, 6ZXG, 6ZXH - PubMed Abstract:
Eukaryotic ribosomes consist of a small 40S and a large 60S subunit that are assembled in a highly coordinated manner. More than 200 factors ensure correct modification, processing and folding of ribosomal RNA and the timely incorporation of ribosomal proteins 1,2 . Small subunit maturation ends in the cytosol, when the final rRNA precursor, 18S-E, is cleaved at site 3 by the endonuclease NOB1 3 . Previous structures of human 40S precursors have shown that NOB1 is kept in an inactive state by its partner PNO1 4 . The final maturation events, including the activation of NOB1 for the decisive rRNA-cleavage step and the mechanisms driving the dissociation of the last biogenesis factors have, however, remained unresolved. Here we report five cryo-electron microscopy structures of human 40S subunit precursors, which describe the compositional and conformational progression during the final steps of 40S assembly. Our structures explain the central role of RIOK1 in the displacement and dissociation of PNO1, which in turn allows conformational changes and activation of the endonuclease NOB1. In addition, we observe two factors, eukaryotic translation initiation factor 1A domain-containing protein (EIF1AD) and leucine-rich repeat-containing protein 47 (LRRC47), which bind to late pre-40S particles near RIOK1 and the central rRNA helix 44. Finally, functional data shows that EIF1AD is required for efficient assembly factor recycling and 18S-E processing. Our results thus enable a detailed understanding of the last steps in 40S formation in human cells and, in addition, provide evidence for principal differences in small ribosomal subunit formation between humans and the model organism Saccharomyces cerevisiae.
- Gene Center Munich, Department of Biochemistry, University of Munich, Munich, Germany.
Organizational Affiliation: