Structural and Functional Insights into the Biofilm-Associated BceF Tyrosine Kinase Domain from Burkholderia cepacia .
Mayer, M., Matiuhin, Y., Nawatha, M., Tabachnikov, O., Fish, I., Schutz, N., Dvir, H., Landau, M.(2021) Biomolecules 11
- PubMed: 34439861
- DOI: https://doi.org/10.3390/biom11081196
- Primary Citation of Related Structures:
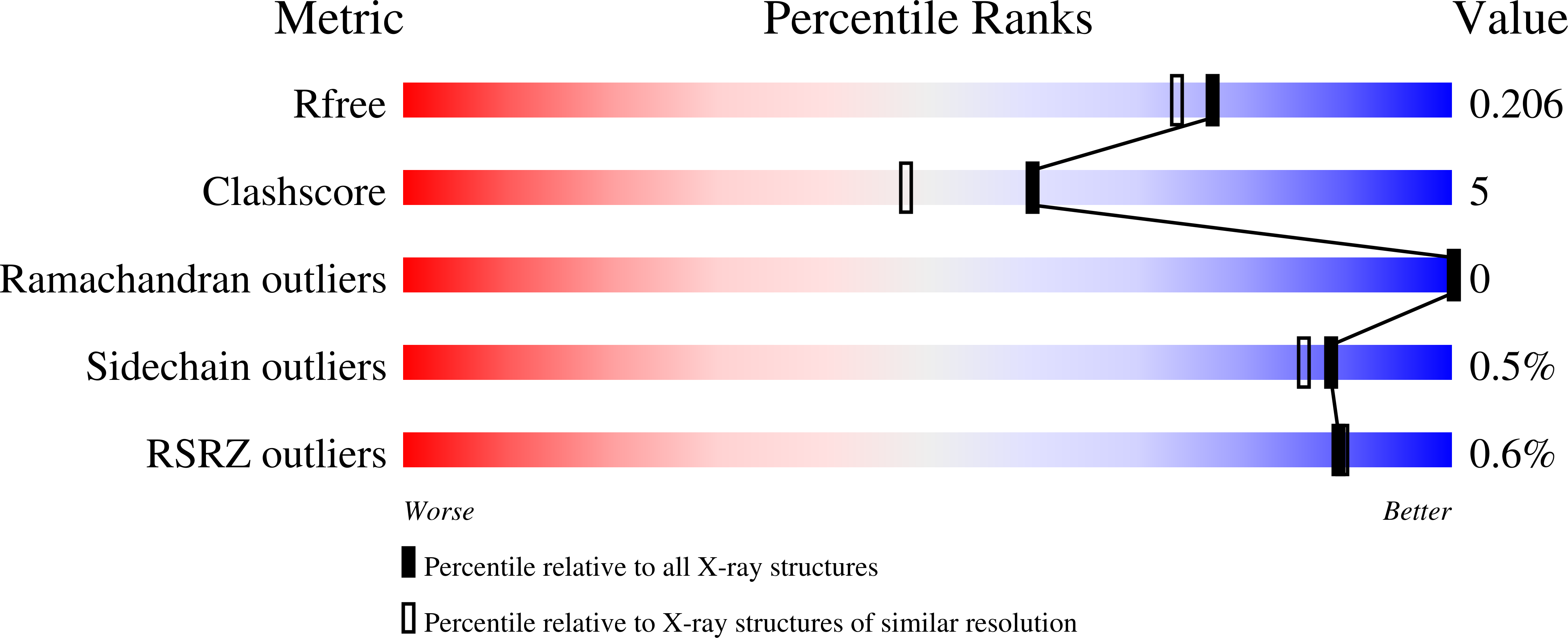
6Z0P - PubMed Abstract:
BceF is a bacterial tyrosine kinase (BY-kinase) from Burkholderia cepacia , a Gram-negative bacterium accountable for respiratory infections in immunocompromised and cystic fibrosis patients. BceF is involved in the production of exopolysaccharides secreted to the biofilm matrix and promotes resistant and aggressive infections. BY-kinases share no homology with mammalian kinases, and thereby offer a means to develop novel and specific antivirulence drugs. Here, we report the crystal structure of the BceF kinase domain at 1.85 Å resolution. The isolated BceF kinase domain is assembled as a dimer in solution and crystallized as a dimer in the asymmetric unit with endogenous adenosine-diphosphate bound at the active sites. The low enzymatic efficiency measured in solution may be explained by the partial obstruction of the active sites at the crystallographic dimer interface. This study provides insights into self-assembly and the specific activity of isolated catalytic domains. Several unique variations around the active site compared to other BY-kinases may allow for structure-based design of specific inhibitors to target Burkholderia cepacia virulence.
Organizational Affiliation:
Department of Biology, Technion-Israel Institute of Technology, Haifa 3200003, Israel.