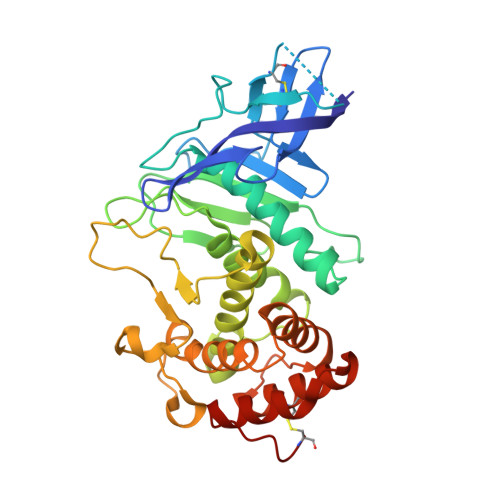
Zinc metalloprotease ProA of Legionella pneumophila increases alveolar septal thickness in human lung tissue explants by collagen IV degradation.
Scheithauer, L., Thiem, S., Schmelz, S., Dellmann, A., Bussow, K., Brouwer, R.M.H.J., Unal, C.M., Blankenfeldt, W., Steinert, M.(2021) Cell Microbiol 23: e13313-e13313
- PubMed: 33491325
- DOI: https://doi.org/10.1111/cmi.13313
- Primary Citation of Related Structures:
6YA1, 6YZE - PubMed Abstract:
ProA is a secreted zinc metalloprotease of Legionella pneumophila causing lung damage in animal models of Legionnaires' disease. Here we demonstrate that ProA promotes infection of human lung tissue explants (HLTEs) and dissect the contribution to cell type specific replication and extracellular virulence mechanisms. For the first time, we reveal that co-incubation of HLTEs with purified ProA causes a significant increase of the alveolar septal thickness. This destruction of connective tissue fibres was further substantiated by collagen IV degradation assays. The moderate attenuation of a proA-negative mutant in A549 epithelial cells and THP-1 macrophages suggests that effects of ProA in tissue mainly result from extracellular activity. Correspondingly, ProA contributes to dissemination and serum resistance of the pathogen, which further expands the versatile substrate spectrum of this thermolysin-like protease. The crystal structure of ProA at 1.48 Å resolution showed high congruence to pseudolysin of Pseudomonas aeruginosa, but revealed deviations in flexible loops, the substrate binding pocket S 1 ' and the repertoire of cofactors, by which ProA can be distinguished from respective homologues. In sum, this work specified virulence features of ProA at different organisational levels by zooming in from histopathological effects in human lung tissue to atomic details of the protease substrate determination.
- Institut für Mikrobiologie, Technische Universität Braunschweig, Braunschweig, Germany.
Organizational Affiliation: