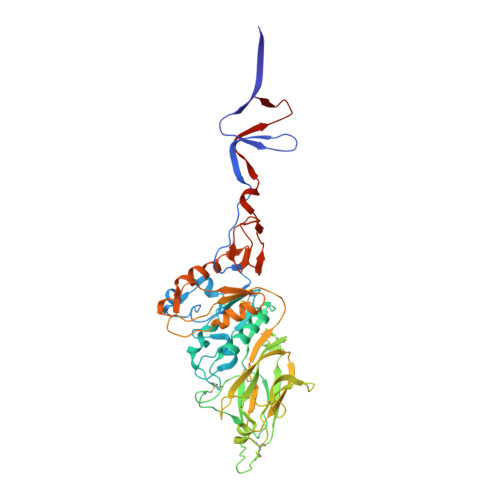
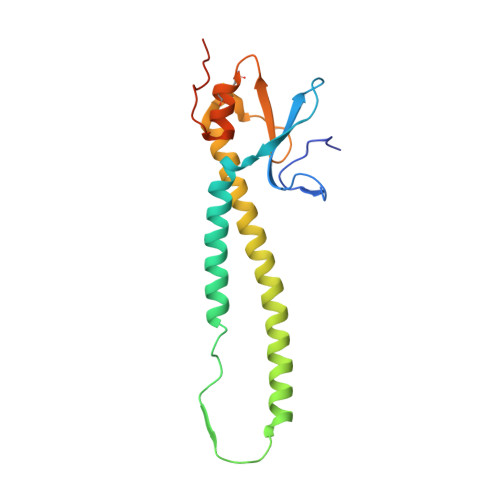
In situ structure and organization of the influenza C virus surface glycoprotein.
Halldorsson, S., Sader, K., Turner, J., Calder, L.J., Rosenthal, P.B.(2021) Nat Commun 12: 1694-1694
- PubMed: 33727554
- DOI: https://doi.org/10.1038/s41467-021-21818-9
- Primary Citation of Related Structures:
6YI5 - PubMed Abstract:
The lipid-enveloped influenza C virus contains a single surface glycoprotein, the haemagglutinin-esterase-fusion (HEF) protein, that mediates receptor binding, receptor destruction, and membrane fusion at the low pH of the endosome. Here we apply electron cryotomography and subtomogram averaging to describe the structural basis for hexagonal lattice formation by HEF on the viral surface. The conformation of the glycoprotein in situ is distinct from the structure of the isolated trimeric ectodomain, showing that a splaying of the membrane distal domains is required to mediate contacts that form the lattice. The splaying of these domains is also coupled to changes in the structure of the stem region which is involved in membrane fusion, thereby linking HEF's membrane fusion conformation with its assembly on the virus surface. The glycoprotein lattice can form independent of other virion components but we show a major role for the matrix layer in particle formation.
- Structural Biology of Cells and Viruses Laboratory, The Francis Crick Institute, London, United Kingdom.
Organizational Affiliation: