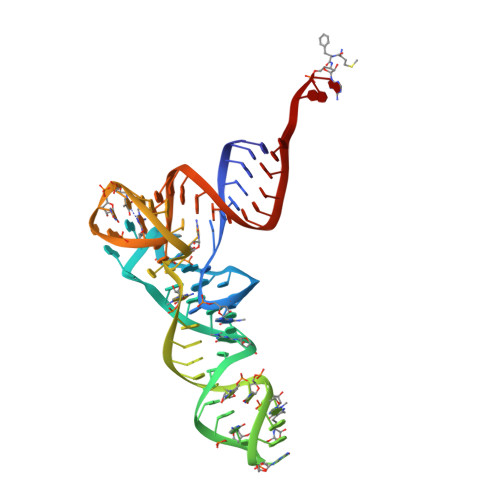
Insights into the improved macrolide inhibitory activity from the high-resolution cryo-EM structure of dirithromycin bound to theE. coli70S ribosome.
Pichkur, E.B., Paleskava, A., Tereshchenkov, A.G., Kasatsky, P., Komarova, E.S., Shiriaev, D.I., Bogdanov, A.A., Dontsova, O.A., Osterman, I.A., Sergiev, P.V., Polikanov, Y.S., Myasnikov, A.G., Konevega, A.L.(2020) RNA 26: 715-723
- PubMed: 32144191
- DOI: https://doi.org/10.1261/rna.073817.119
- Primary Citation of Related Structures:
6XZ7, 6XZA, 6XZB - PubMed Abstract:
Macrolides are one of the most successful and widely used classes of antibacterials, which kill or stop the growth of pathogenic bacteria by binding near the active site of the ribosome and interfering with protein synthesis. Dirithromycin is a derivative of the prototype macrolide erythromycin with additional hydrophobic side chain. In our recent study, we have discovered that the side chain of dirithromycin forms lone pair-π stacking interaction with the aromatic imidazole ring of the His69 residue in ribosomal protein uL4 of the Thermus thermophilus 70S ribosome. In the current work, we found that neither the presence of the side chain, nor the additional contact with the ribosome, improve the binding affinity of dirithromycin to the ribosome. Nevertheless, we found that dirithromycin is a more potent inhibitor of in vitro protein synthesis in comparison with its parent compound, erythromycin. Using high-resolution cryo-electron microscopy, we determined the structure of the dirithromycin bound to the translating Escherichia coli 70S ribosome, which suggests that the better inhibitory properties of the drug could be rationalized by the side chain of dirithromycin pointing into the lumen of the nascent peptide exit tunnel, where it can interfere with the normal passage of the growing polypeptide chain.
- Petersburg Nuclear Physics Institute named by B.P. Konstantinov of NRC "Kurchatov Institute," Gatchina, 188300, Russia.
Organizational Affiliation: