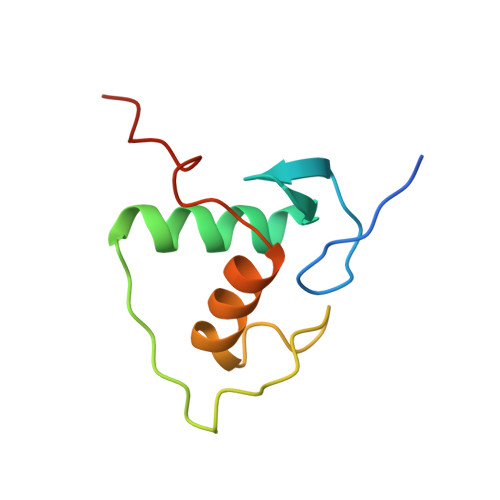
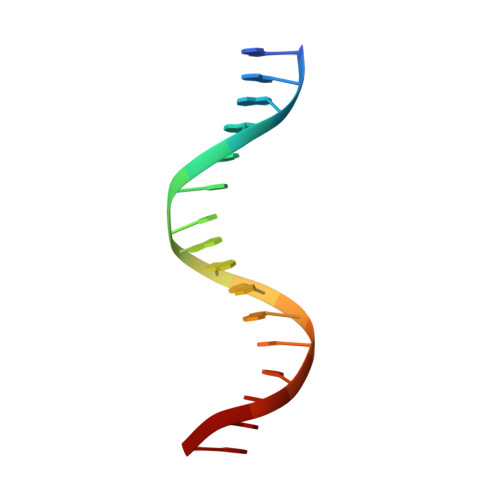
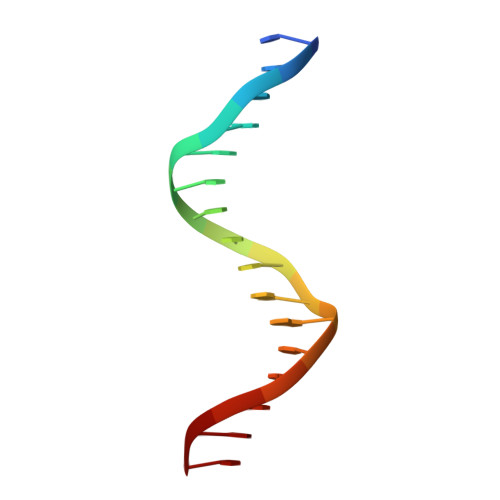
Structural basis for DNA recognition and allosteric control of the retinoic acid receptors RAR-RXR.
Osz, J., McEwen, A.G., Bourguet, M., Przybilla, F., Peluso-Iltis, C., Poussin-Courmontagne, P., Mely, Y., Cianferani, S., Jeffries, C.M., Svergun, D.I., Rochel, N.(2020) Nucleic Acids Res 48: 9969-9985
- PubMed: 32974652
- DOI: https://doi.org/10.1093/nar/gkaa697
- Primary Citation of Related Structures:
6XWG, 6XWH - PubMed Abstract:
Retinoic acid receptors (RARs) as a functional heterodimer with retinoid X receptors (RXRs), bind a diverse series of RA-response elements (RAREs) in regulated genes. Among them, the non-canonical DR0 elements are bound by RXR-RAR with comparable affinities to DR5 elements but DR0 elements do not act transcriptionally as independent RAREs. In this work, we present structural insights for the recognition of DR5 and DR0 elements by RXR-RAR heterodimer using x-ray crystallography, small angle x-ray scattering, and hydrogen/deuterium exchange coupled to mass spectrometry. We solved the crystal structures of RXR-RAR DNA-binding domain in complex with the Rarb2 DR5 and RXR-RXR DNA-binding domain in complex with Hoxb13 DR0. While cooperative binding was observed on DR5, the two molecules bound non-cooperatively on DR0 on opposite sides of the DNA. In addition, our data unveil the structural organization and dynamics of the multi-domain RXR-RAR DNA complexes providing evidence for DNA-dependent allosteric communication between domains. Differential binding modes between DR0 and DR5 were observed leading to differences in conformation and structural dynamics of the multi-domain RXR-RAR DNA complexes. These results reveal that the topological organization of the RAR binding element confer regulatory information by modulating the overall topology and structural dynamics of the RXR-RAR heterodimers.
- Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), Illkirch, France.
Organizational Affiliation: