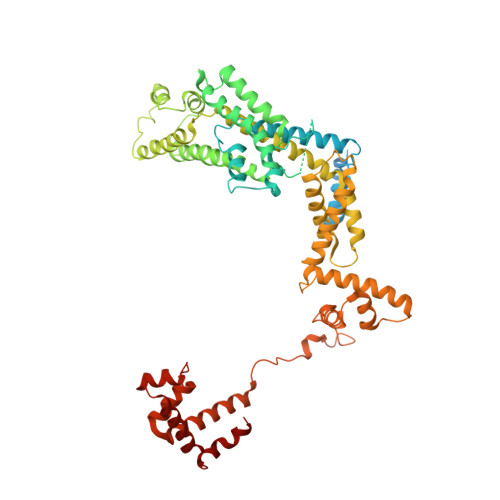
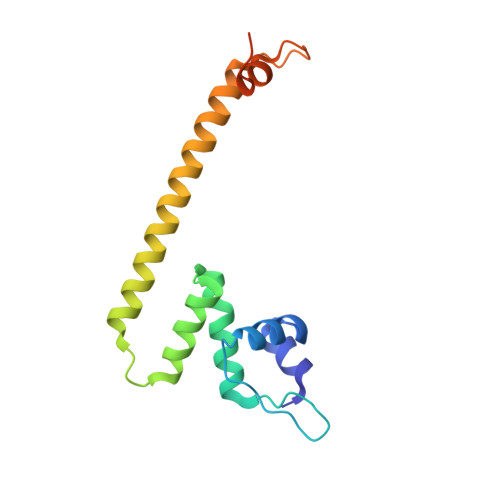
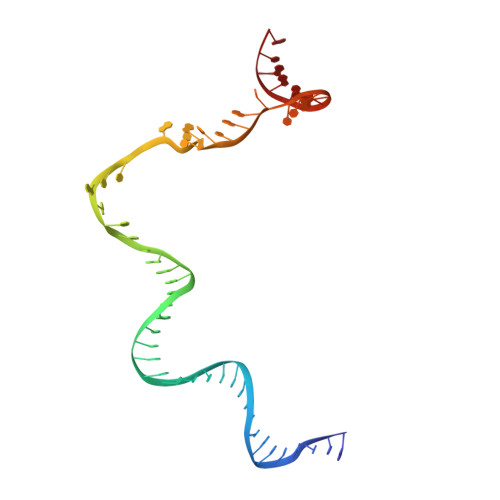
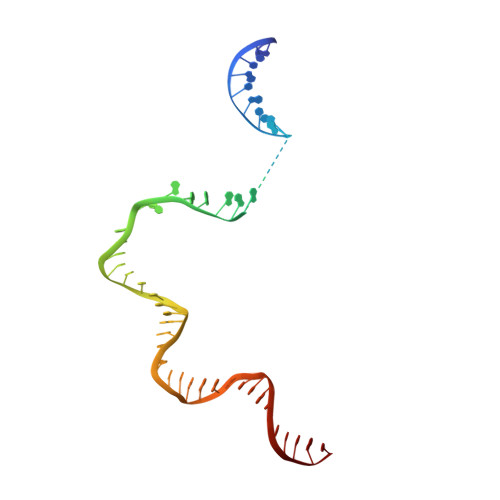
Structural basis of copper-efflux-regulator-dependent transcription activation.
Shi, W., Zhang, B., Jiang, Y., Liu, C., Zhou, W., Chen, M., Yang, Y., Hu, Y., Liu, B.(2021) iScience 24: 102449-102449
- PubMed: 34113812
- DOI: https://doi.org/10.1016/j.isci.2021.102449
- Primary Citation of Related Structures:
6XH7, 6XH8 - PubMed Abstract:
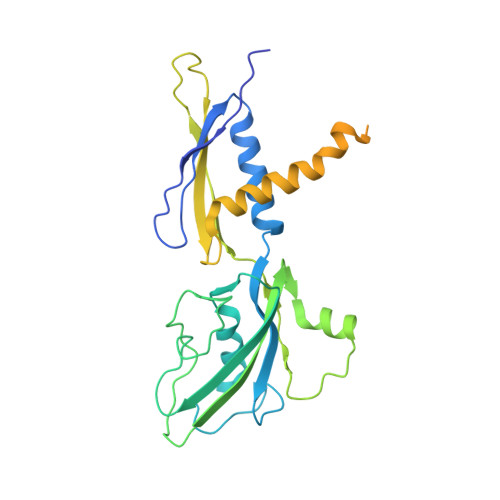
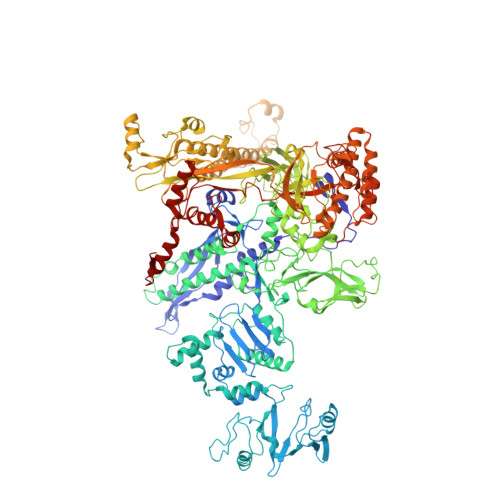
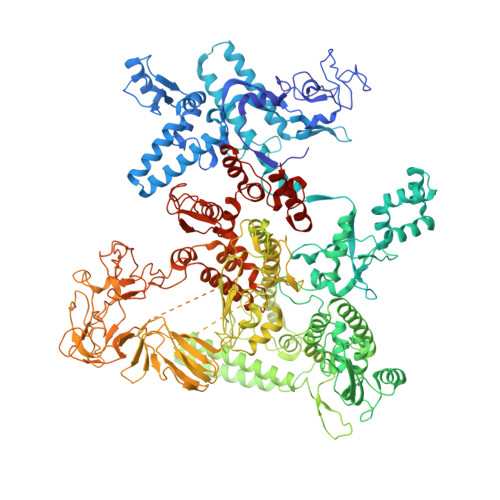
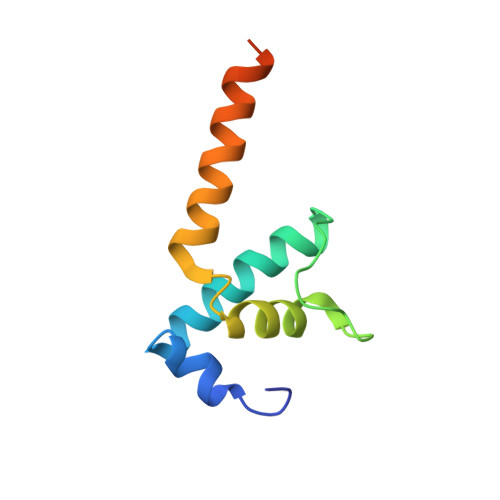
The copper efflux regulator (CueR), a representative member of mercury resistance regulator (MerR) family metalloregulators, controls expression of copper homeostasis-regulating genes in bacteria. The mechanism of transcription activation by CueR and other MerR family regulators is bending the spacer domain of promoter DNA. Here, we report the cryo-EM structures of the intact CueR-dependent transcription activation complexes. The structures show that CueR dimer bends the 19-bp promoter spacer to realign the -35 and -10 elements for recognition by σ 70 -RNA polymerase holoenzyme and reveal a previously unreported interaction between the DNA-binding domain (DBD) from one CueR subunit and the σ 70 nonconserved region (σNCR). Functional studies have shown that the CueR-σNCR interaction plays an auxiliary role in CueR-dependent transcription, assisting the activation mechanism of bending promoter DNA by CueR dimer. Because DBDs are highly conserved in sequence and structure, this transcription-activating mechanism could be generally used by MerR family regulators.
- Section of Transcription & Gene Regulation, The Hormel Institute, University of Minnesota, Austin, MN, USA.
Organizational Affiliation: