Caspase-7 in complex with elongated ketomethylene inhibitor
Solania, A., Xu, J.H., Wolan, D.W.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Caspase-7 | A, C [auth B] | 198 | Homo sapiens | Mutation(s): 0 Gene Names: CASP7, MCH3 EC: 3.4.22.60 | 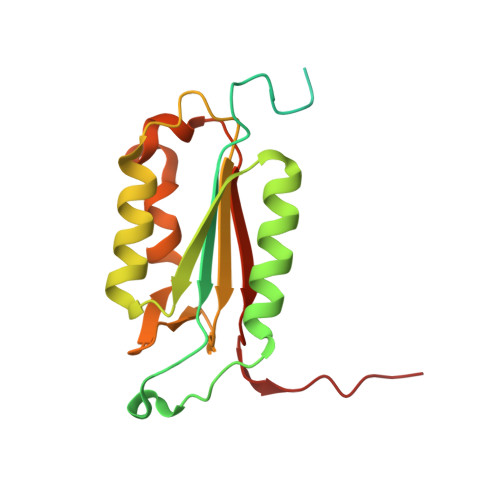 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P55210 (Homo sapiens) Explore P55210 Go to UniProtKB: P55210 | |||||
PHAROS: P55210 GTEx: ENSG00000165806 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P55210 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Caspase-7 | B [auth C], D | 113 | Homo sapiens | Mutation(s): 0 Gene Names: CASP7, MCH3 EC: 3.4.22.60 | 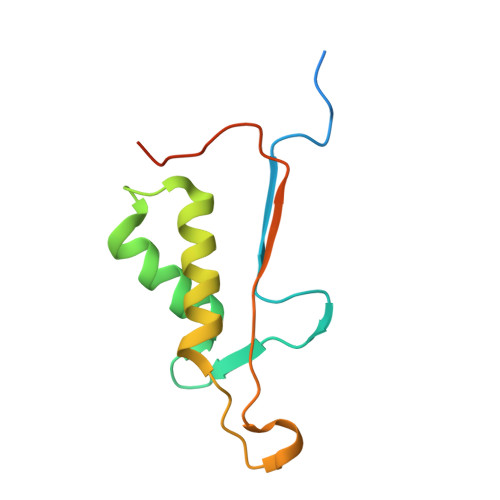 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P55210 (Homo sapiens) Explore P55210 Go to UniProtKB: P55210 | |||||
PHAROS: P55210 GTEx: ENSG00000165806 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P55210 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ketomethylene inhibitor | 8 | synthetic construct | Mutation(s): 0 | 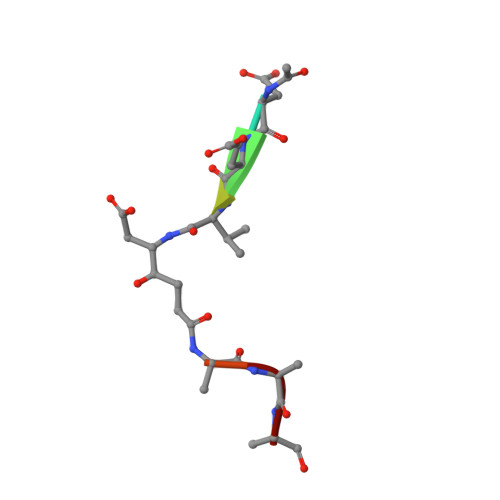 | |
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 88.296 | α = 90 |
| b = 88.296 | β = 90 |
| c = 187.28 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | 5R21AI112796 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | 1R01GM118382 |