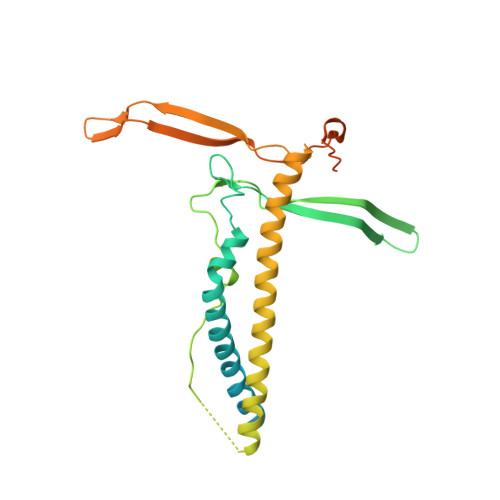
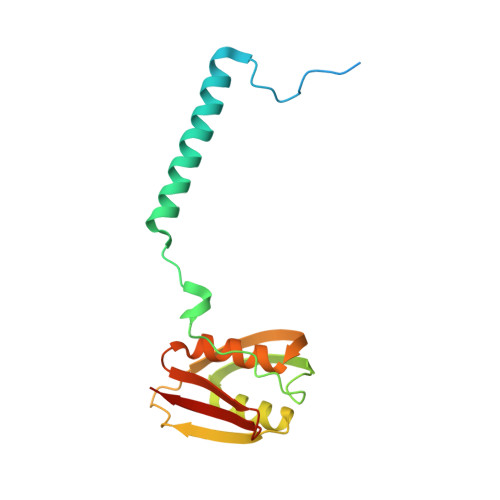
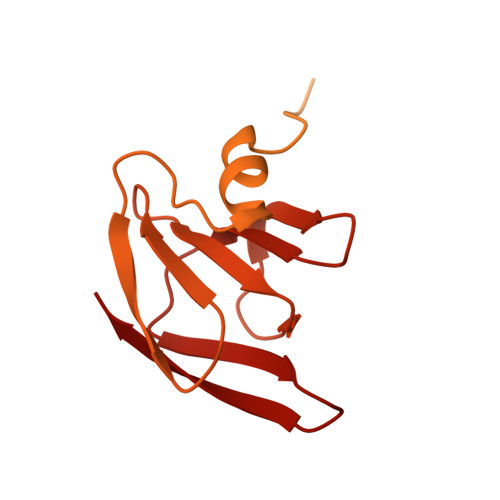
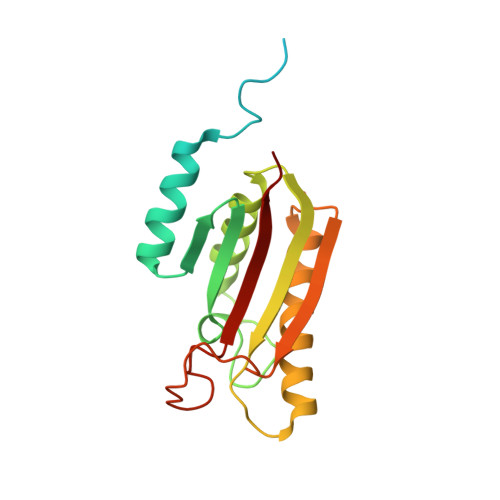
Structural analysis of the Legionella pneumophila Dot/Icm type IV secretion system core complex.
Durie, C.L., Sheedlo, M.J., Chung, J.M., Byrne, B.G., Su, M., Knight, T., Swanson, M., Lacy, D.B., Ohi, M.D.(2020) Elife 9
- PubMed: 32876045
- DOI: https://doi.org/10.7554/eLife.59530
- Primary Citation of Related Structures:
6X62, 6X64, 6X65, 6X66 - PubMed Abstract:
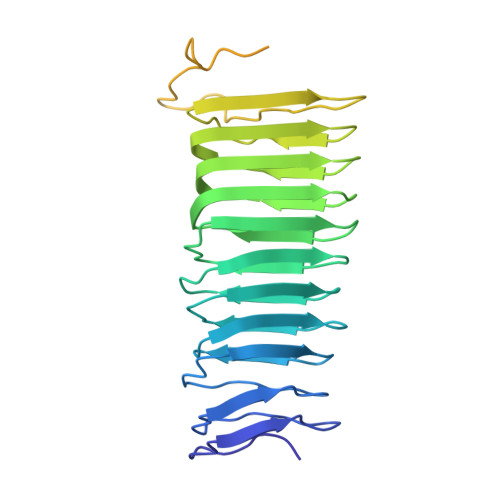
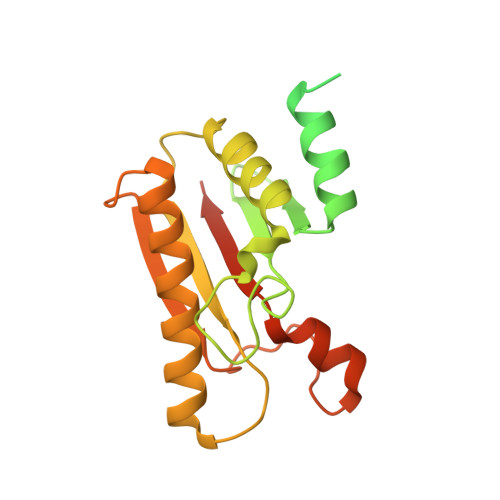
Legionella pneumophila is an opportunistic pathogen that causes the potentially fatal pneumonia Legionnaires' Disease. This infection and subsequent pathology require the Dot/Icm Type IV Secretion System (T4SS) to deliver effector proteins into host cells. Compared to prototypical T4SSs, the Dot/Icm assembly is much larger, containing ~27 different components including a core complex reported to be composed of five proteins: DotC, DotD, DotF, DotG, and DotH. Using single particle cryo-electron microscopy (cryo-EM), we report reconstructions of the core complex of the Dot/Icm T4SS that includes a symmetry mismatch between distinct structural features of the outer membrane cap (OMC) and periplasmic ring (PR). We present models of known core complex proteins, DotC, DotD, and DotH, and two structurally similar proteins within the core complex, DotK and Lpg0657. This analysis reveals the stoichiometry and contact interfaces between the key proteins of the Dot/Icm T4SS core complex and provides a framework for understanding a complex molecular machine.
- Life Sciences Institute, University of Michigan, Ann Arbor, United States.
Organizational Affiliation: