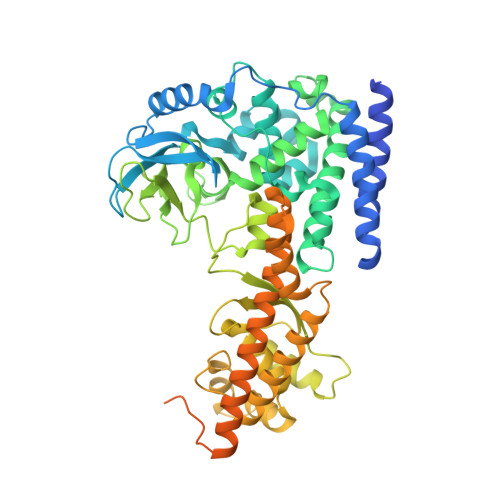
Characterization of SETD3 methyltransferase-mediated protein methionine methylation.
Dai, S., Holt, M.V., Horton, J.R., Woodcock, C.B., Patel, A., Zhang, X., Young, N.L., Wilkinson, A.W., Cheng, X.(2020) J Biological Chem 295: 10901-10910
- PubMed: 32503840
- DOI: https://doi.org/10.1074/jbc.RA120.014072
- Primary Citation of Related Structures:
6WK1, 6WK2 - PubMed Abstract:
Most characterized protein methylation events encompass arginine and lysine N -methylation, and only a few cases of protein methionine thiomethylation have been reported. Newly discovered oncohistone mutations include lysine-to-methionine substitutions at positions 27 and 36 of histone H3.3. In these instances, the methionine substitution localizes to the active-site pocket of the corresponding histone lysine methyltransferase, thereby inhibiting the respective transmethylation activity. SET domain-containing 3 (SETD3) is a protein ( i.e. actin) histidine methyltransferase. Here, we generated an actin variant in which the histidine target of SETD3 was substituted with methionine. As for previously characterized histone SET domain proteins, the methionine substitution substantially (76-fold) increased binding affinity for SETD3 and inhibited SETD3 activity on histidine. Unexpectedly, SETD3 was active on the substituted methionine, generating S -methylmethionine in the context of actin peptide. The ternary structure of SETD3 in complex with the methionine-containing actin peptide at 1.9 Å resolution revealed that the hydrophobic thioether side chain is packed by the aromatic rings of Tyr 312 and Trp 273 , as well as the hydrocarbon side chain of Ile 310 Our results suggest that placing methionine properly in the active site-within close proximity to and in line with the incoming methyl group of SAM-would allow some SET domain proteins to selectively methylate methionine in proteins.
- Department of Epigenetics and Molecular Carcinogenesis, University of Texas M. D. Anderson Cancer Center, Houston, Texas, USA.
Organizational Affiliation: